 Found 47 hits with Last Name = 'kapadnis' and Initial = 'p'
Found 47 hits with Last Name = 'kapadnis' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362181
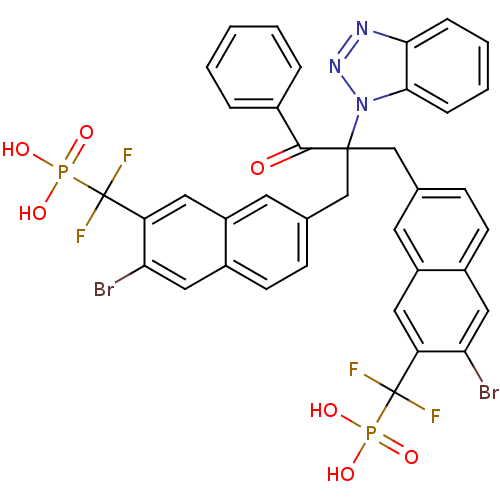
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362195
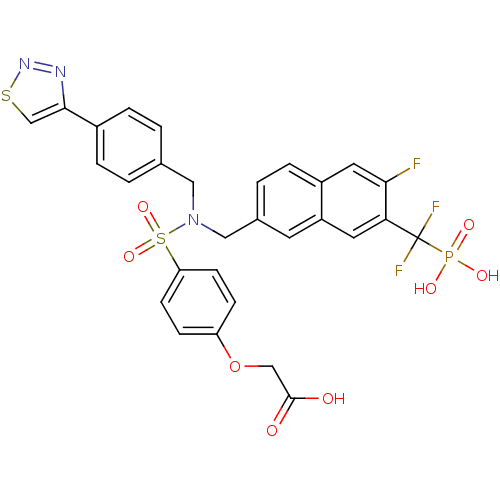
(CHEMBL1938828)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C29H23F3N3O8PS2/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)44(38,39)40)15-35(14-18-1-4-20(5-2-18)27-17-45-34-33-27)46(41,42)24-9-7-23(8-10-24)43-16-28(36)37/h1-13,17H,14-16H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362196
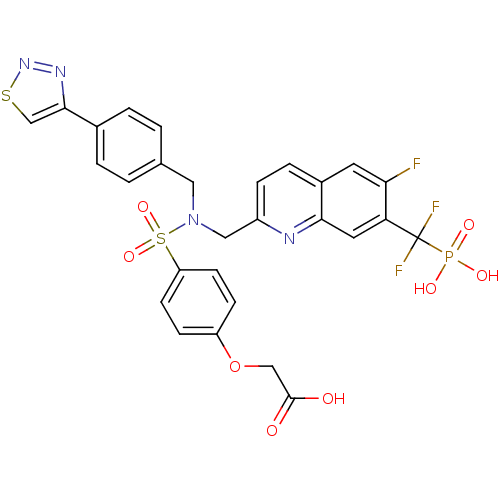
(CHEMBL1938829)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2n1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C28H22F3N4O8PS2/c29-24-11-19-5-6-20(32-25(19)12-23(24)28(30,31)44(38,39)40)14-35(13-17-1-3-18(4-2-17)26-16-45-34-33-26)46(41,42)22-9-7-21(8-10-22)43-15-27(36)37/h1-12,16H,13-15H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362191
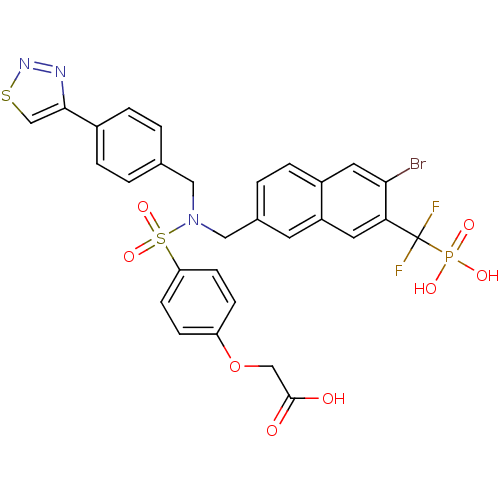
(CHEMBL1938824)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(Br)c(cc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C29H23BrF2N3O8PS2/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)44(38,39)40)15-35(14-18-1-4-20(5-2-18)27-17-45-34-33-27)46(41,42)24-9-7-23(8-10-24)43-16-28(36)37/h1-13,17H,14-16H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362192
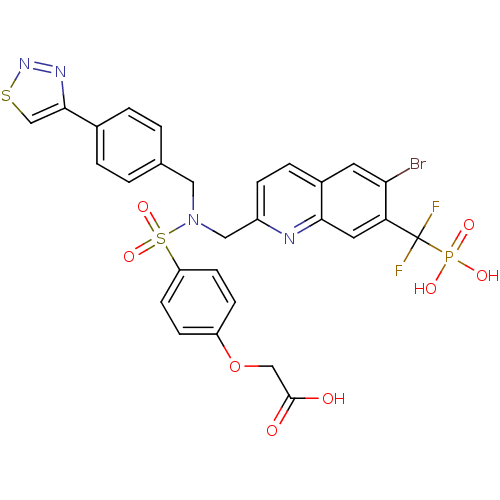
(CHEMBL1938825)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(Br)c(cc2n1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C28H22BrF2N4O8PS2/c29-24-11-19-5-6-20(32-25(19)12-23(24)28(30,31)44(38,39)40)14-35(13-17-1-3-18(4-2-17)26-16-45-34-33-26)46(41,42)22-9-7-21(8-10-22)43-15-27(36)37/h1-12,16H,13-15H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362186
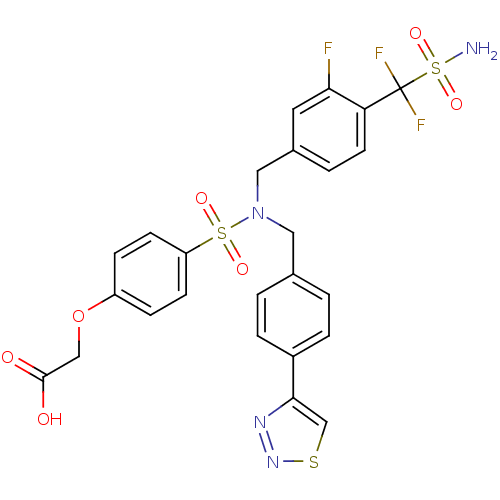
(CHEMBL1938819)Show SMILES NS(=O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1F Show InChI InChI=1S/C25H21F3N4O7S3/c26-22-11-17(3-10-21(22)25(27,28)42(29,37)38)13-32(12-16-1-4-18(5-2-16)23-15-40-31-30-23)41(35,36)20-8-6-19(7-9-20)39-14-24(33)34/h1-11,15H,12-14H2,(H,33,34)(H2,29,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362197
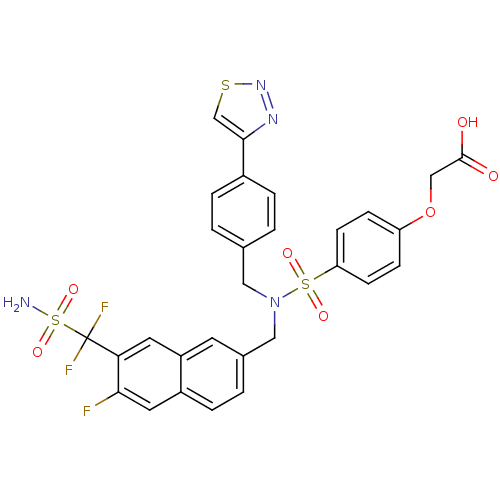
(CHEMBL1938830)Show SMILES NS(=O)(=O)C(F)(F)c1cc2cc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1F Show InChI InChI=1S/C29H23F3N4O7S3/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)46(33,41)42)15-36(14-18-1-4-20(5-2-18)27-17-44-35-34-27)45(39,40)24-9-7-23(8-10-24)43-16-28(37)38/h1-13,17H,14-16H2,(H,37,38)(H2,33,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362183
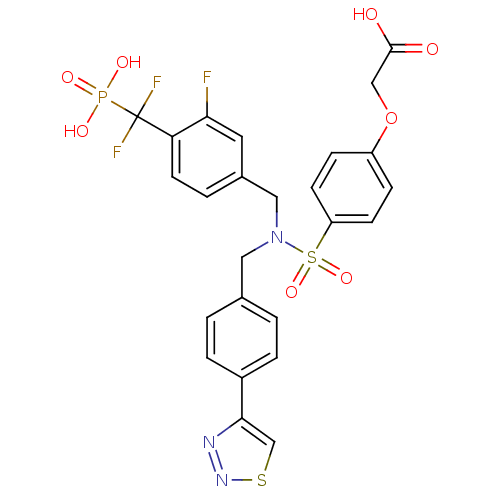
(CHEMBL1938816)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(F)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21F3N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362198
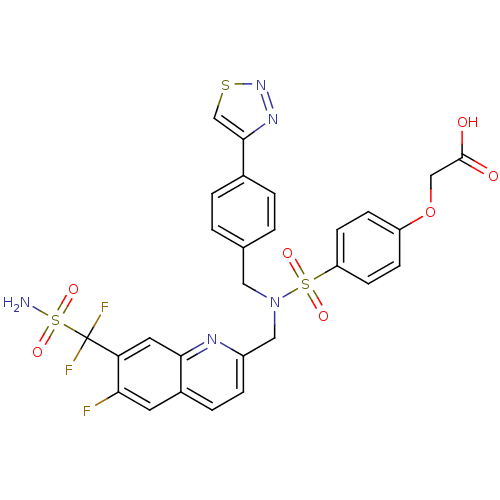
(CHEMBL1938831)Show SMILES NS(=O)(=O)C(F)(F)c1cc2nc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1F Show InChI InChI=1S/C28H22F3N5O7S3/c29-24-11-19-5-6-20(33-25(19)12-23(24)28(30,31)46(32,41)42)14-36(13-17-1-3-18(4-2-17)26-16-44-35-34-26)45(39,40)22-9-7-21(8-10-22)43-15-27(37)38/h1-12,16H,13-15H2,(H,37,38)(H2,32,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362193
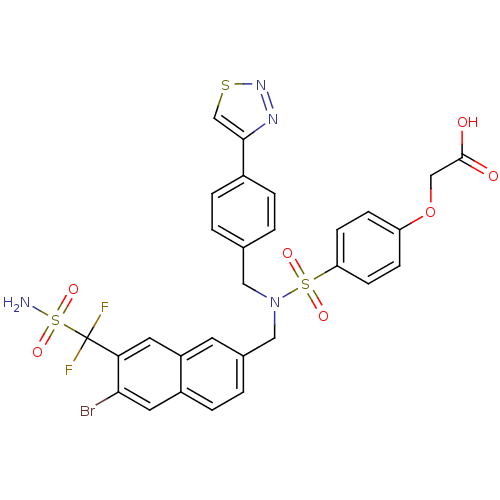
(CHEMBL1938826)Show SMILES NS(=O)(=O)C(F)(F)c1cc2cc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1Br Show InChI InChI=1S/C29H23BrF2N4O7S3/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)46(33,41)42)15-36(14-18-1-4-20(5-2-18)27-17-44-35-34-27)45(39,40)24-9-7-23(8-10-24)43-16-28(37)38/h1-13,17H,14-16H2,(H,37,38)(H2,33,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362194
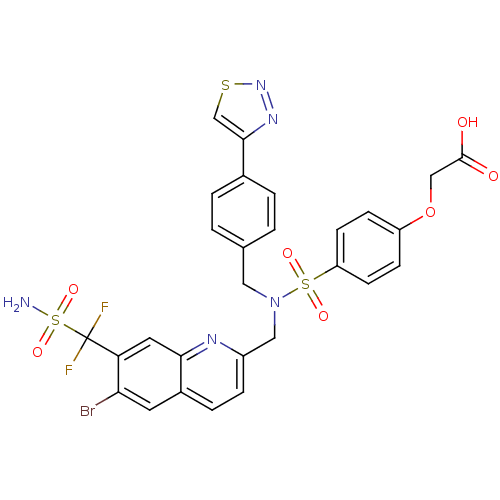
(CHEMBL1938827)Show SMILES NS(=O)(=O)C(F)(F)c1cc2nc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1Br Show InChI InChI=1S/C28H22BrF2N5O7S3/c29-24-11-19-5-6-20(33-25(19)12-23(24)28(30,31)46(32,41)42)14-36(13-17-1-3-18(4-2-17)26-16-44-35-34-26)45(39,40)22-9-7-21(8-10-22)43-15-27(37)38/h1-12,16H,13-15H2,(H,37,38)(H2,32,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362185
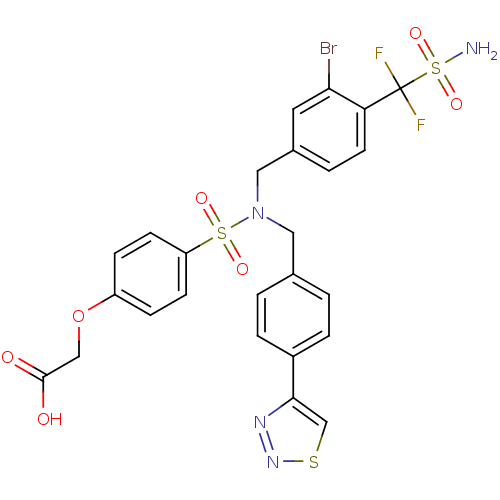
(CHEMBL1938818)Show SMILES NS(=O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1Br Show InChI InChI=1S/C25H21BrF2N4O7S3/c26-22-11-17(3-10-21(22)25(27,28)42(29,37)38)13-32(12-16-1-4-18(5-2-16)23-15-40-31-30-23)41(35,36)20-8-6-19(7-9-20)39-14-24(33)34/h1-11,15H,12-14H2,(H,33,34)(H2,29,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171096
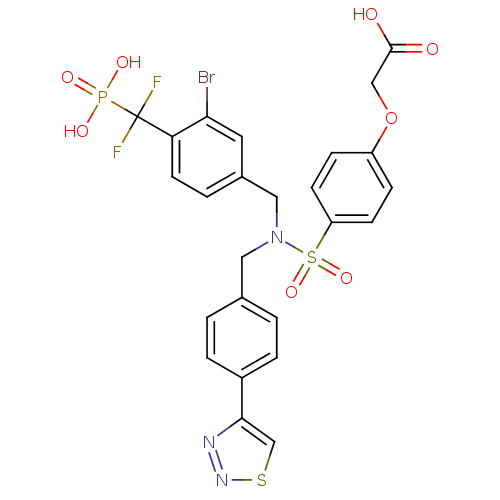
(CHEMBL371929 | {4-[[3-Bromo-4-(difluoro-phosphono-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362184
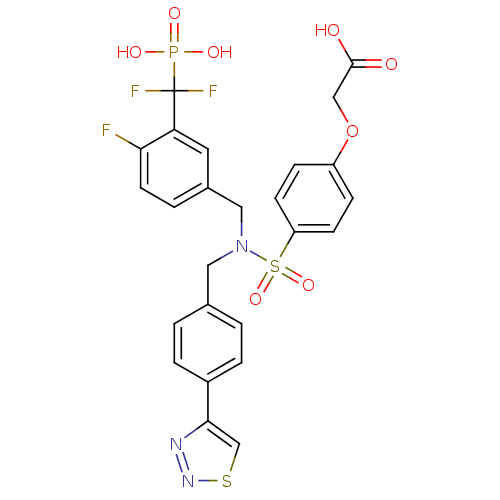
(CHEMBL1938817)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(F)c(c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21F3N3O8PS2/c26-22-10-3-17(11-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362182
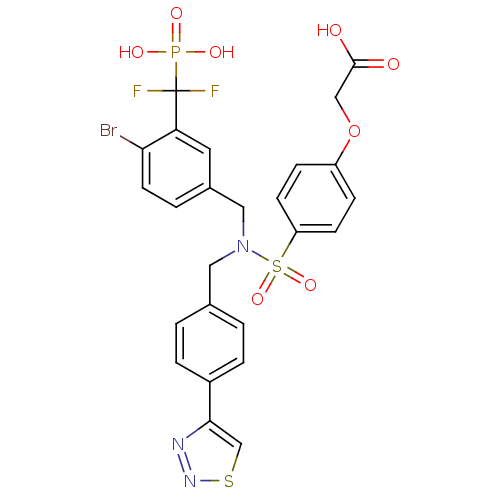
(CHEMBL1938815)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(Br)c(c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-10-3-17(11-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362190
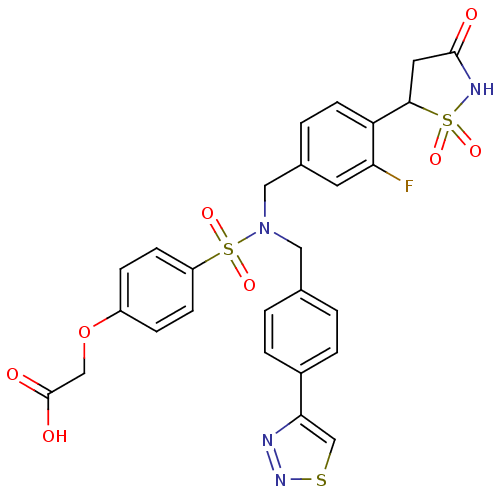
(CHEMBL1938823)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(C2CC(=O)NS2(=O)=O)c(F)c1 Show InChI InChI=1S/C27H23FN4O8S3/c28-23-11-18(3-10-22(23)25-12-26(33)30-42(25,36)37)14-32(13-17-1-4-19(5-2-17)24-16-41-31-29-24)43(38,39)21-8-6-20(7-9-21)40-15-27(34)35/h1-11,16,25H,12-15H2,(H,30,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362188
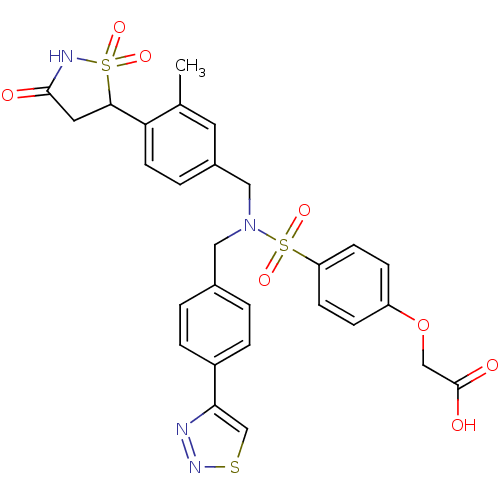
(CHEMBL1938821)Show SMILES Cc1cc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)ccc1C1CC(=O)NS1(=O)=O Show InChI InChI=1S/C28H26N4O8S3/c1-18-12-20(4-11-24(18)26-13-27(33)30-42(26,36)37)15-32(14-19-2-5-21(6-3-19)25-17-41-31-29-25)43(38,39)23-9-7-22(8-10-23)40-16-28(34)35/h2-12,17,26H,13-16H2,1H3,(H,30,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362189
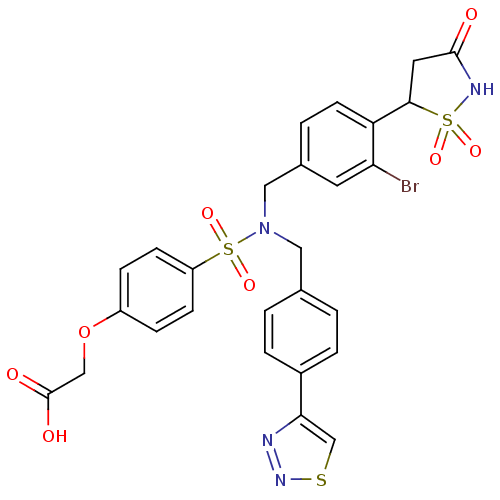
(CHEMBL1938822)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(C2CC(=O)NS2(=O)=O)c(Br)c1 Show InChI InChI=1S/C27H23BrN4O8S3/c28-23-11-18(3-10-22(23)25-12-26(33)30-42(25,36)37)14-32(13-17-1-4-19(5-2-17)24-16-41-31-29-24)43(38,39)21-8-6-20(7-9-21)40-15-27(34)35/h1-11,16,25H,12-15H2,(H,30,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362187
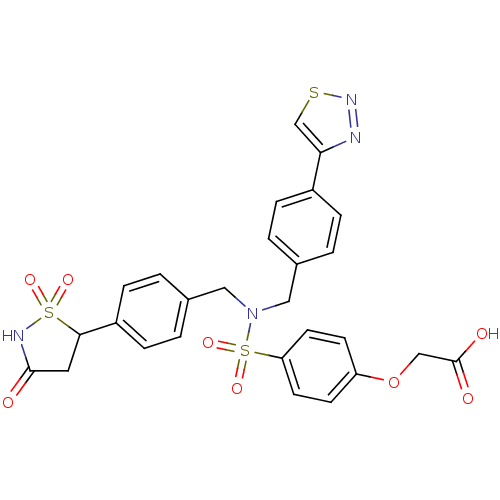
(CHEMBL1938820)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)Cc1ccc(cc1)-c1csnn1 Show InChI InChI=1S/C27H24N4O8S3/c32-26-13-25(41(35,36)29-26)21-7-3-19(4-8-21)15-31(14-18-1-5-20(6-2-18)24-17-40-30-28-24)42(37,38)23-11-9-22(10-12-23)39-16-27(33)34/h1-12,17,25H,13-16H2,(H,29,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50362181

(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50362186

(CHEMBL1938819)Show SMILES NS(=O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1F Show InChI InChI=1S/C25H21F3N4O7S3/c26-22-11-17(3-10-21(22)25(27,28)42(29,37)38)13-32(12-16-1-4-18(5-2-16)23-15-40-31-30-23)41(35,36)20-8-6-19(7-9-20)39-14-24(33)34/h1-11,15H,12-14H2,(H,33,34)(H2,29,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50171096

(CHEMBL371929 | {4-[[3-Bromo-4-(difluoro-phosphono-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50362195

(CHEMBL1938828)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C29H23F3N3O8PS2/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)44(38,39)40)15-35(14-18-1-4-20(5-2-18)27-17-45-34-33-27)46(41,42)24-9-7-23(8-10-24)43-16-28(36)37/h1-13,17H,14-16H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50362183

(CHEMBL1938816)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(F)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21F3N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50362196

(CHEMBL1938829)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2n1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C28H22F3N4O8PS2/c29-24-11-19-5-6-20(32-25(19)12-23(24)28(30,31)44(38,39)40)14-35(13-17-1-3-18(4-2-17)26-16-45-34-33-26)46(41,42)22-9-7-21(8-10-22)43-15-27(36)37/h1-12,16H,13-15H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259048

(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 Show InChI InChI=1S/C19H17Cl3N4O2S/c1-11-17(19(27)24-25-6-8-28-9-7-25)23-26(14-3-2-12(20)10-13(14)21)18(11)15-4-5-16(22)29-15/h2-5,10H,6-9H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.43E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259093
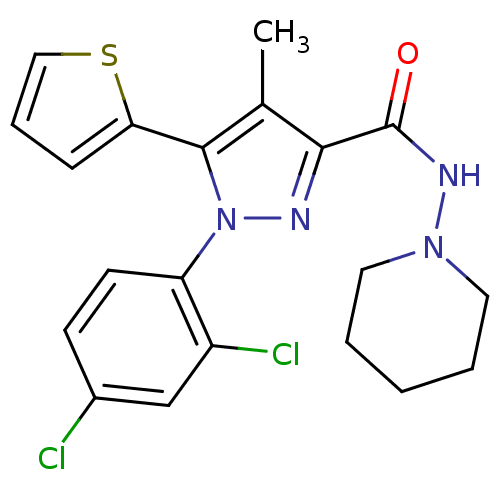
(1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...)Show SMILES Cc1c(nn(c1-c1cccs1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H20Cl2N4OS/c1-13-18(20(27)24-25-9-3-2-4-10-25)23-26(19(13)17-6-5-11-28-17)16-8-7-14(21)12-15(16)22/h5-8,11-12H,2-4,9-10H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.55E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253755
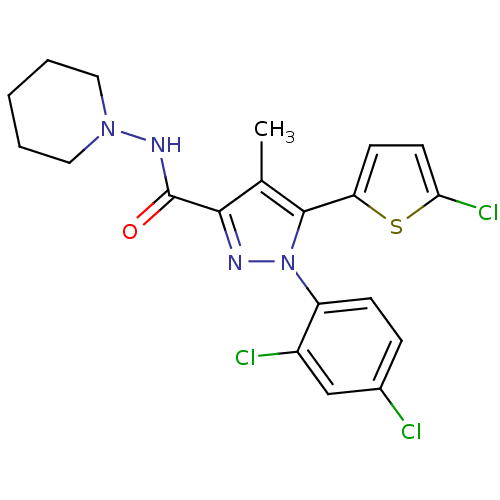
(5-(5-Chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19Cl3N4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(21)11-14(15)22)19(12)16-7-8-17(23)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253755

(5-(5-Chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19Cl3N4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(21)11-14(15)22)19(12)16-7-8-17(23)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253704
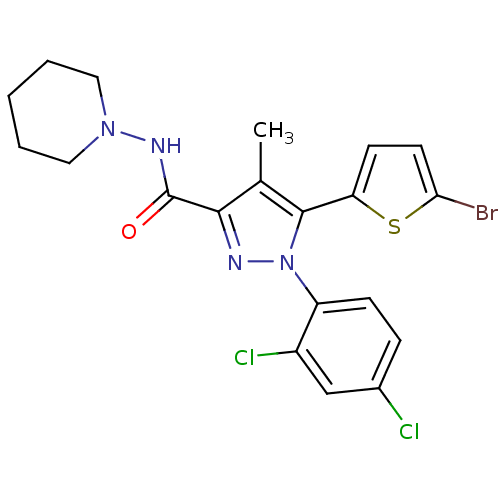
(5-(5-bromothiophen-2-yl)-1-(2,4-dichlorophenyl)-4-...)Show SMILES Cc1c(nn(c1-c1ccc(Br)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19BrCl2N4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(22)11-14(15)23)19(12)16-7-8-17(21)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253704

(5-(5-bromothiophen-2-yl)-1-(2,4-dichlorophenyl)-4-...)Show SMILES Cc1c(nn(c1-c1ccc(Br)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19BrCl2N4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(22)11-14(15)23)19(12)16-7-8-17(21)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259006

(1-(2,4-dichlorophenyl)-5-(5-iodothiophen-2-yl)-4-m...)Show SMILES Cc1c(nn(c1-c1ccc(I)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19Cl2IN4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(21)11-14(15)22)19(12)16-7-8-17(23)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259006

(1-(2,4-dichlorophenyl)-5-(5-iodothiophen-2-yl)-4-m...)Show SMILES Cc1c(nn(c1-c1ccc(I)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H19Cl2IN4OS/c1-12-18(20(28)25-26-9-3-2-4-10-26)24-27(15-6-5-13(21)11-14(15)22)19(12)16-7-8-17(23)29-16/h5-8,11H,2-4,9-10H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259091
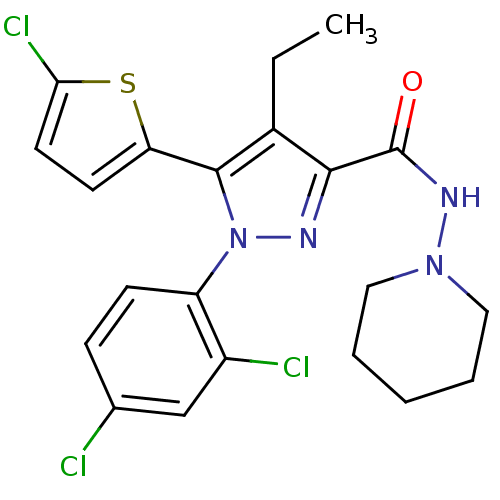
(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES CCc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C21H21Cl3N4OS/c1-2-14-19(21(29)26-27-10-4-3-5-11-27)25-28(16-7-6-13(22)12-15(16)23)20(14)17-8-9-18(24)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259091

(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES CCc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C21H21Cl3N4OS/c1-2-14-19(21(29)26-27-10-4-3-5-11-27)25-28(16-7-6-13(22)12-15(16)23)20(14)17-8-9-18(24)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259092

(5-(5-bromothiophen-2-yl)-1-(2,4-dichlorophenyl)-4-...)Show SMILES CCc1c(nn(c1-c1ccc(Br)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C21H21BrCl2N4OS/c1-2-14-19(21(29)26-27-10-4-3-5-11-27)25-28(16-7-6-13(23)12-15(16)24)20(14)17-8-9-18(22)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259092

(5-(5-bromothiophen-2-yl)-1-(2,4-dichlorophenyl)-4-...)Show SMILES CCc1c(nn(c1-c1ccc(Br)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C21H21BrCl2N4OS/c1-2-14-19(21(29)26-27-10-4-3-5-11-27)25-28(16-7-6-13(23)12-15(16)24)20(14)17-8-9-18(22)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 510 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259047
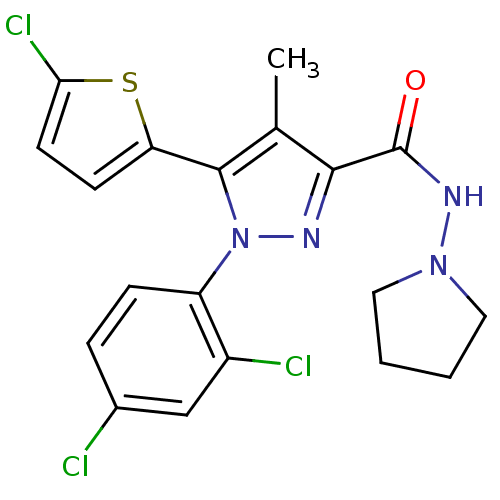
(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCC1 Show InChI InChI=1S/C19H17Cl3N4OS/c1-11-17(19(27)24-25-8-2-3-9-25)23-26(14-5-4-12(20)10-13(14)21)18(11)15-6-7-16(22)28-15/h4-7,10H,2-3,8-9H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259047

(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCC1 Show InChI InChI=1S/C19H17Cl3N4OS/c1-11-17(19(27)24-25-8-2-3-9-25)23-26(14-5-4-12(20)10-13(14)21)18(11)15-6-7-16(22)28-15/h4-7,10H,2-3,8-9H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253756
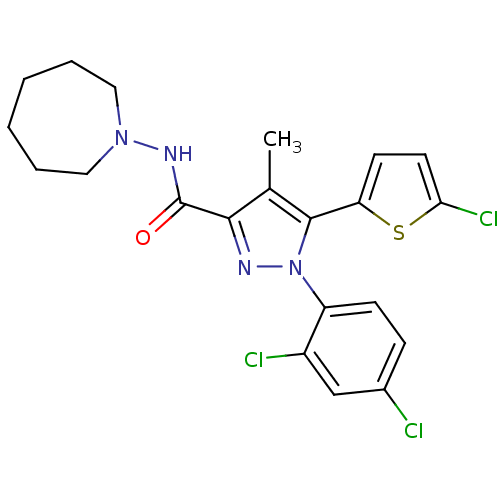
(CHEMBL461862 | N-(Azepan-1-yl)-5-(5-chlorothiophen...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C21H21Cl3N4OS/c1-13-19(21(29)26-27-10-4-2-3-5-11-27)25-28(16-7-6-14(22)12-15(16)23)20(13)17-8-9-18(24)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253756

(CHEMBL461862 | N-(Azepan-1-yl)-5-(5-chlorothiophen...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C21H21Cl3N4OS/c1-13-19(21(29)26-27-10-4-2-3-5-11-27)25-28(16-7-6-14(22)12-15(16)23)20(13)17-8-9-18(24)30-17/h6-9,12H,2-5,10-11H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259048

(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-4...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 Show InChI InChI=1S/C19H17Cl3N4O2S/c1-11-17(19(27)24-25-6-8-28-9-7-25)23-26(14-3-2-12(20)10-13(14)21)18(11)15-4-5-16(22)29-15/h2-5,10H,6-9H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259049
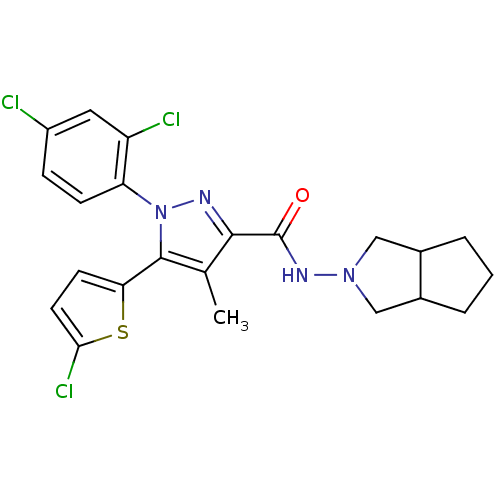
(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-N...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CC2CCCC2C1 Show InChI InChI=1S/C22H21Cl3N4OS/c1-12-20(22(30)27-28-10-13-3-2-4-14(13)11-28)26-29(17-6-5-15(23)9-16(17)24)21(12)18-7-8-19(25)31-18/h5-9,13-14H,2-4,10-11H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50259049

(5-(5-chlorothiophen-2-yl)-1-(2,4-dichlorophenyl)-N...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)s1)-c1ccc(Cl)cc1Cl)C(=O)NN1CC2CCCC2C1 Show InChI InChI=1S/C22H21Cl3N4OS/c1-12-20(22(30)27-28-10-13-3-2-4-14(13)11-28)26-29(17-6-5-15(23)9-16(17)24)21(12)18-7-8-19(25)31-18/h5-9,13-14H,2-4,10-11H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259093

(1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...)Show SMILES Cc1c(nn(c1-c1cccs1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C20H20Cl2N4OS/c1-13-18(20(27)24-25-9-3-2-4-10-25)23-26(19(13)17-6-5-11-28-17)16-8-7-14(21)12-15(16)22/h5-8,11-12H,2-4,9-10H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activity |
Bioorg Med Chem Lett 19: 2546-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.046
BindingDB Entry DOI: 10.7270/Q2TB16SP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data