| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM50281636 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_160728 |
|---|
| Ki | 65±n/a nM |
|---|
| Citation |  Sawyer, T; Fisher, J; Hester, J; Smith, C; Tomasselli, A; Tarpley, W; Burton, P; Hui, J; McQuade, T; Conradi, R; Bradford, V; Liu, L; Kinner, J; Tustin, J; Alexander, D; Harrison, A; Emmert, D; Staples, D; Maggiora, L; Zhang, Y; Poorman, R; Dunna, B; Rao, C; Scarborough, P; Lowther, W; Craik, C; DeCamp, D; Moon, J; Howe, W; Heinrikson, R Peptidomimetic inhibitors of human immunodeficiency virus protease (HIV-PR): Design, enzyme binding and selectivity, antiviral efficacy, and cell permeability properties Bioorg Med Chem Lett3:819-824 (1993) Article Sawyer, T; Fisher, J; Hester, J; Smith, C; Tomasselli, A; Tarpley, W; Burton, P; Hui, J; McQuade, T; Conradi, R; Bradford, V; Liu, L; Kinner, J; Tustin, J; Alexander, D; Harrison, A; Emmert, D; Staples, D; Maggiora, L; Zhang, Y; Poorman, R; Dunna, B; Rao, C; Scarborough, P; Lowther, W; Craik, C; DeCamp, D; Moon, J; Howe, W; Heinrikson, R Peptidomimetic inhibitors of human immunodeficiency virus protease (HIV-PR): Design, enzyme binding and selectivity, antiviral efficacy, and cell permeability properties Bioorg Med Chem Lett3:819-824 (1993) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | Human rhinovirus A protease | Human rhinovirus B 3A protease |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 44361.04 |
|---|
| Organism: | Human rhinovirus B |
|---|
| Description: | ChEMBL_158953 |
|---|
| Residue: | 401 |
|---|
| Sequence: | AFRPCNVNTKIGNAKCCPFVCGKAVTFKDRSTCSTYNLSSSLHHILEEDKRRRQVVDVMS
AIFQGPISLDAPPPPAIADLLQSVRTPRVIKYCQIIMGHPAECQVERDLNIANSIIAIIA
NIISIAGIIFVIYKLFCSLQGPYSGEPKPKTKVPERRVVAQGPEEEFGRSILKNNTCVIT
TGNGKFTGLGIHDRILIIPTHADPGREVQVNGVHTKVLDSYDLYNRDGVKLEITVIQLDR
NEKFRDIRKYIPETEDDYPECNLALSANQDEPTIIKVGDVVSYGNILLSGNQTARMLKYN
YPTKSGYCGGVLYKIGQILGIHVGGNGRDGFSAMLLRSYFTGQIKVNKHATECGLPDIQT
IHTPSKTKLQPSVFYDVFPGSKEPAVLTDNDPRLEVNFKEA
|
|
|
|---|
| BDBM50281636 |
|---|
| n/a |
|---|
| Name | BDBM50281636 |
|---|
| Synonyms: | (S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-4-{(S)-2-methyl-1-[(pyridin-2-ylmethyl)-carbamoyl]-butylcarbamoyl}-hexyl)-2-[(quinoline-2-carbonyl)-amino]-succinamide | CHEMBL167514 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H57N7O6 |
|---|
| Mol. Mass. | 743.9346 |
|---|
| SMILES | CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 |
|---|
| Structure | 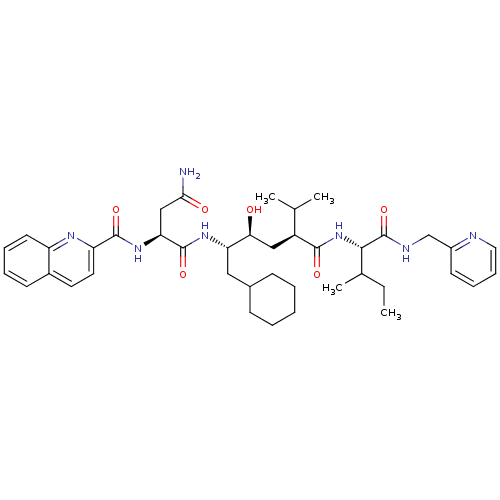
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sawyer, T; Fisher, J; Hester, J; Smith, C; Tomasselli, A; Tarpley, W; Burton, P; Hui, J; McQuade, T; Conradi, R; Bradford, V; Liu, L; Kinner, J; Tustin, J; Alexander, D; Harrison, A; Emmert, D; Staples, D; Maggiora, L; Zhang, Y; Poorman, R; Dunna, B; Rao, C; Scarborough, P; Lowther, W; Craik, C; DeCamp, D; Moon, J; Howe, W; Heinrikson, R Peptidomimetic inhibitors of human immunodeficiency virus protease (HIV-PR): Design, enzyme binding and selectivity, antiviral efficacy, and cell permeability properties Bioorg Med Chem Lett3:819-824 (1993) Article
Sawyer, T; Fisher, J; Hester, J; Smith, C; Tomasselli, A; Tarpley, W; Burton, P; Hui, J; McQuade, T; Conradi, R; Bradford, V; Liu, L; Kinner, J; Tustin, J; Alexander, D; Harrison, A; Emmert, D; Staples, D; Maggiora, L; Zhang, Y; Poorman, R; Dunna, B; Rao, C; Scarborough, P; Lowther, W; Craik, C; DeCamp, D; Moon, J; Howe, W; Heinrikson, R Peptidomimetic inhibitors of human immunodeficiency virus protease (HIV-PR): Design, enzyme binding and selectivity, antiviral efficacy, and cell permeability properties Bioorg Med Chem Lett3:819-824 (1993) Article