 Found 236 hits with Last Name = 'kinner' and Initial = 'j'
Found 236 hits with Last Name = 'kinner' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50212827
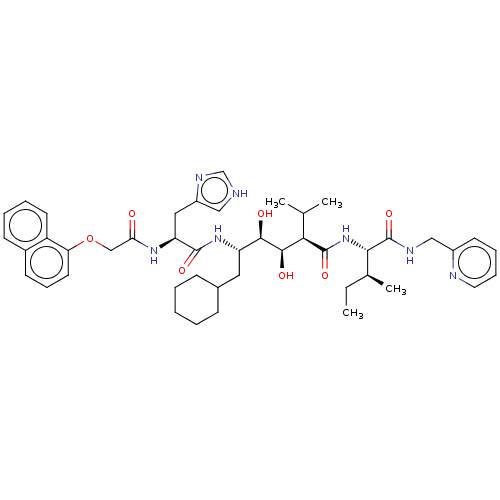
(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50281636
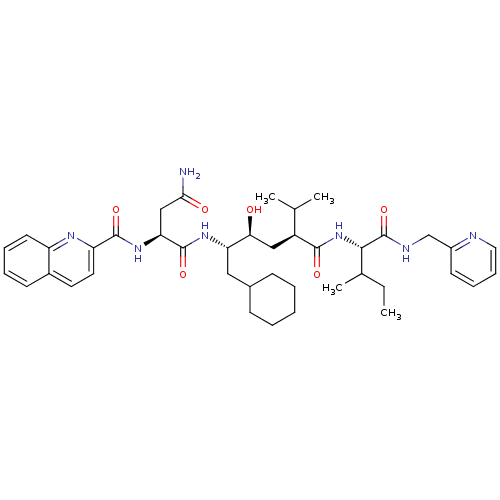
((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281638
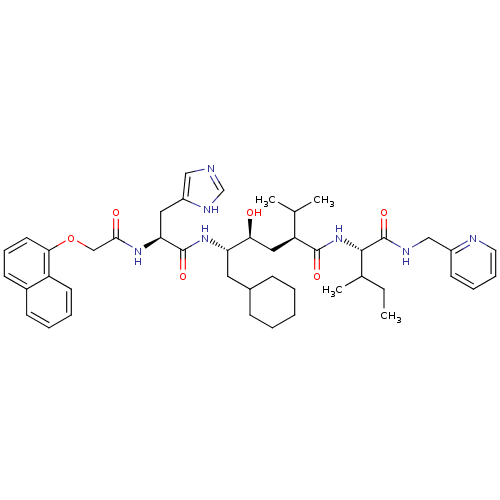
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281641
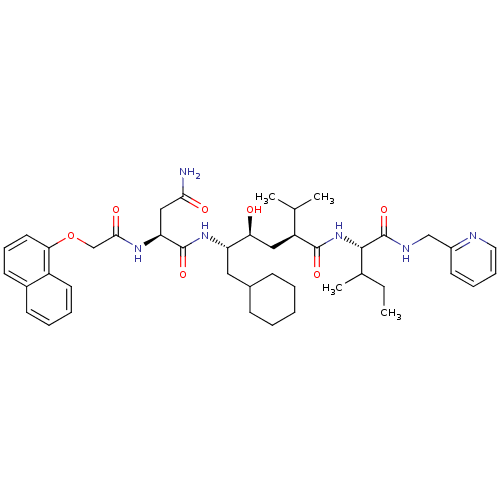
((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C43H60N6O7/c1-5-28(4)40(43(55)46-25-31-18-11-12-21-45-31)49-41(53)33(27(2)3)23-36(50)34(22-29-14-7-6-8-15-29)48-42(54)35(24-38(44)51)47-39(52)26-56-37-20-13-17-30-16-9-10-19-32(30)37/h9-13,16-21,27-29,33-36,40,50H,5-8,14-15,22-26H2,1-4H3,(H2,44,51)(H,46,55)(H,47,52)(H,48,54)(H,49,53)/t28?,33-,34-,35-,36-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281640
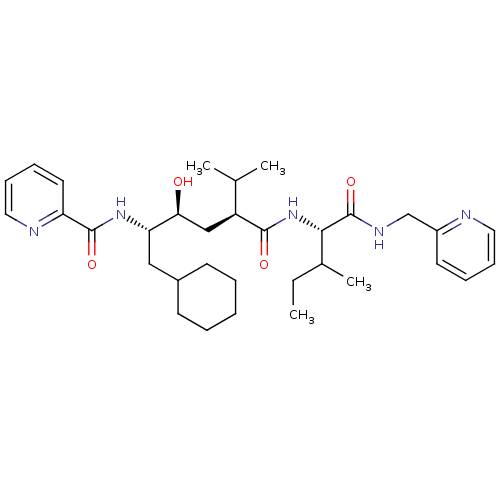
(CHEMBL433729 | Pyridine-2-carboxylic acid ((1S,2S,...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccn1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C33H49N5O4/c1-5-23(4)30(33(42)36-21-25-15-9-11-17-34-25)38-31(40)26(22(2)3)20-29(39)28(19-24-13-7-6-8-14-24)37-32(41)27-16-10-12-18-35-27/h9-12,15-18,22-24,26,28-30,39H,5-8,13-14,19-21H2,1-4H3,(H,36,42)(H,37,41)(H,38,40)/t23?,26-,28-,29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281642
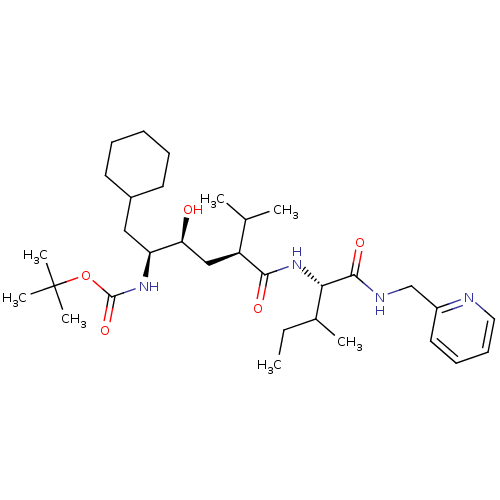
(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281651
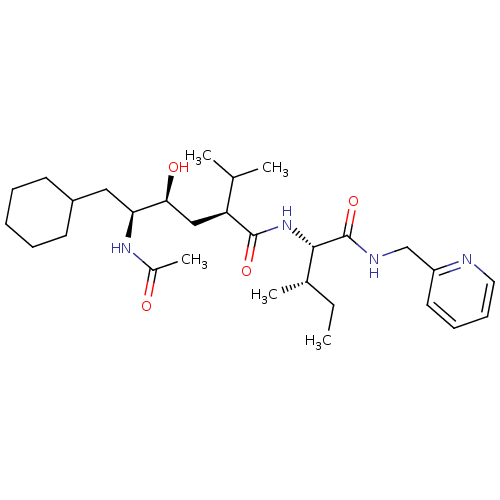
((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(C)=O)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C29H48N4O4/c1-6-20(4)27(29(37)31-18-23-14-10-11-15-30-23)33-28(36)24(19(2)3)17-26(35)25(32-21(5)34)16-22-12-8-7-9-13-22/h10-11,14-15,19-20,22,24-27,35H,6-9,12-13,16-18H2,1-5H3,(H,31,37)(H,32,34)(H,33,36)/t20-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281648
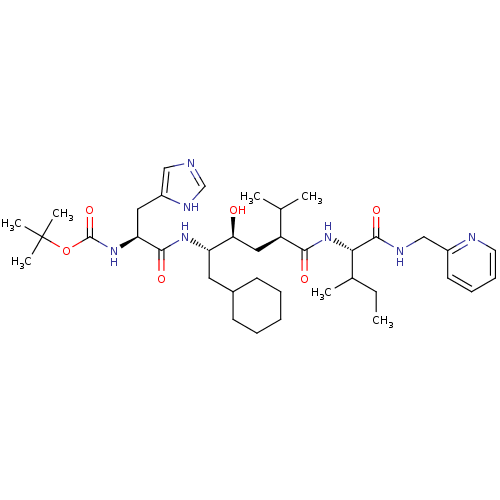
(CHEMBL166640 | [(S)-1-((1S,2S,4S)-1-Cyclohexylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C38H61N7O6/c1-8-25(4)33(36(49)41-22-27-16-12-13-17-40-27)45-34(47)29(24(2)3)20-32(46)30(18-26-14-10-9-11-15-26)43-35(48)31(19-28-21-39-23-42-28)44-37(50)51-38(5,6)7/h12-13,16-17,21,23-26,29-33,46H,8-11,14-15,18-20,22H2,1-7H3,(H,39,42)(H,41,49)(H,43,48)(H,44,50)(H,45,47)/t25?,29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281644
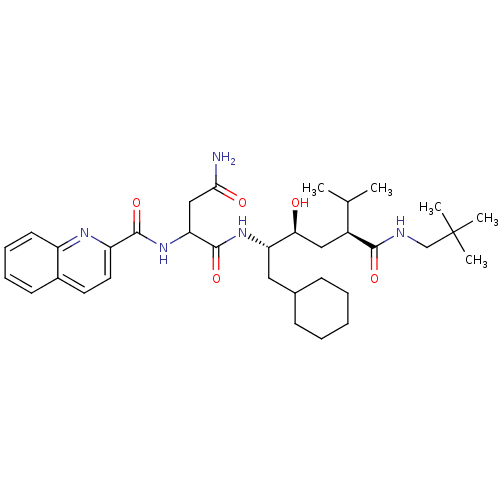
(CHEMBL355867 | N*1*-[(1S,2S,4S)-1-Cyclohexylmethyl...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(=O)NCC(C)(C)C Show InChI InChI=1S/C34H51N5O5/c1-21(2)24(31(42)36-20-34(3,4)5)18-29(40)27(17-22-11-7-6-8-12-22)38-33(44)28(19-30(35)41)39-32(43)26-16-15-23-13-9-10-14-25(23)37-26/h9-10,13-16,21-22,24,27-29,40H,6-8,11-12,17-20H2,1-5H3,(H2,35,41)(H,36,42)(H,38,44)(H,39,43)/t24-,27-,28?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281643
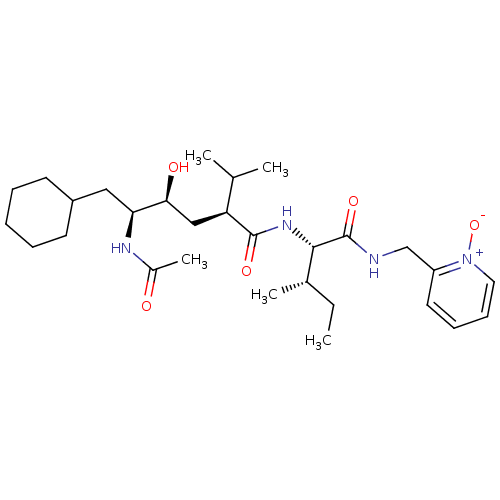
((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(C)=O)C(C)C)C(=O)NCc1cccc[n+]1[O-] Show InChI InChI=1S/C29H48N4O5/c1-6-20(4)27(29(37)30-18-23-14-10-11-15-33(23)38)32-28(36)24(19(2)3)17-26(35)25(31-21(5)34)16-22-12-8-7-9-13-22/h10-11,14-15,19-20,22,24-27,35H,6-9,12-13,16-18H2,1-5H3,(H,30,37)(H,31,34)(H,32,36)/t20-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of HIV-2 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281652
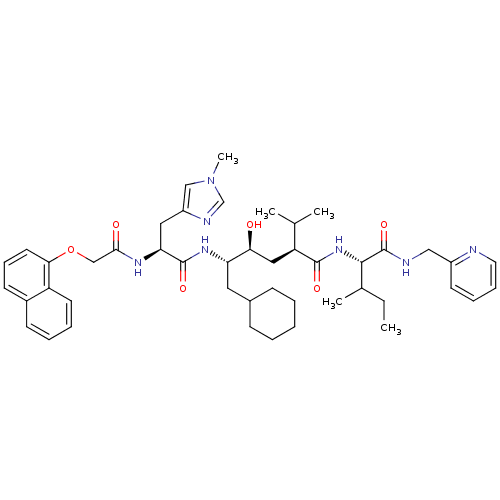
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-2-isopropyl-5-{(...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cn(C)cn1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C46H63N7O6/c1-6-31(4)43(46(58)48-26-34-19-12-13-22-47-34)52-44(56)37(30(2)3)25-40(54)38(23-32-15-8-7-9-16-32)51-45(57)39(24-35-27-53(5)29-49-35)50-42(55)28-59-41-21-14-18-33-17-10-11-20-36(33)41/h10-14,17-22,27,29-32,37-40,43,54H,6-9,15-16,23-26,28H2,1-5H3,(H,48,58)(H,50,55)(H,51,57)(H,52,56)/t31?,37-,38-,39-,40-,43-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281639
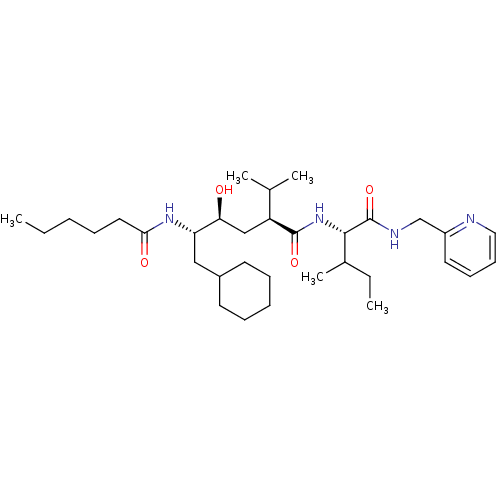
((2S,4S,5S)-6-Cyclohexyl-5-hexanoylamino-4-hydroxy-...)Show SMILES CCCCCC(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N[C@@H](C(C)CC)C(=O)NCc1ccccn1 Show InChI InChI=1S/C33H56N4O4/c1-6-8-10-18-30(39)36-28(20-25-15-11-9-12-16-25)29(38)21-27(23(3)4)32(40)37-31(24(5)7-2)33(41)35-22-26-17-13-14-19-34-26/h13-14,17,19,23-25,27-29,31,38H,6-12,15-16,18,20-22H2,1-5H3,(H,35,41)(H,36,39)(H,37,40)/t24?,27-,28-,29-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin E
(Homo sapiens (Human)) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281637
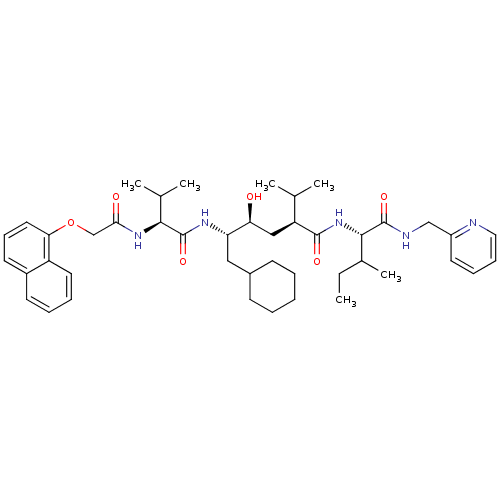
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-2-isopropyl-5-{(...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)COc1cccc2ccccc12)C(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C44H63N5O6/c1-7-30(6)41(43(53)46-26-33-20-13-14-23-45-33)49-42(52)35(28(2)3)25-37(50)36(24-31-16-9-8-10-17-31)47-44(54)40(29(4)5)48-39(51)27-55-38-22-15-19-32-18-11-12-21-34(32)38/h11-15,18-23,28-31,35-37,40-41,50H,7-10,16-17,24-27H2,1-6H3,(H,46,53)(H,47,54)(H,48,51)(H,49,52)/t30?,35-,36-,37-,40-,41-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281646
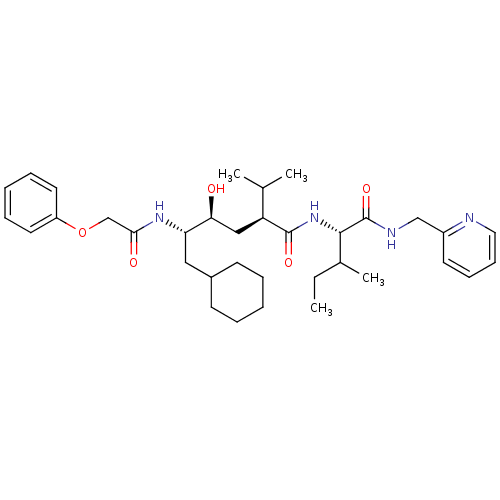
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-2-isopropyl-5-(2...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)COc1ccccc1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C35H52N4O5/c1-5-25(4)33(35(43)37-22-27-16-12-13-19-36-27)39-34(42)29(24(2)3)21-31(40)30(20-26-14-8-6-9-15-26)38-32(41)23-44-28-17-10-7-11-18-28/h7,10-13,16-19,24-26,29-31,33,40H,5-6,8-9,14-15,20-23H2,1-4H3,(H,37,43)(H,38,41)(H,39,42)/t25?,29-,30-,31-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of HIV-2 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281645
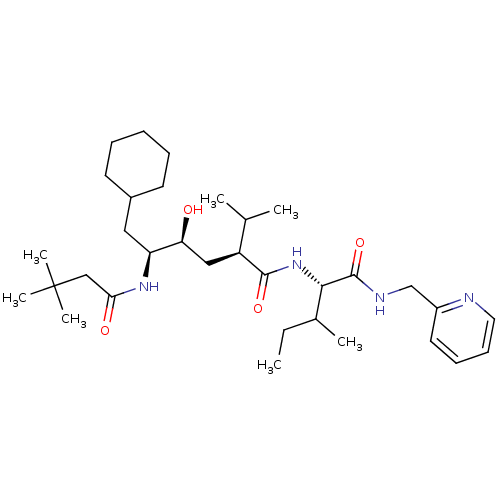
((2S,4S,5S)-6-Cyclohexyl-5-(3,3-dimethyl-butyrylami...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C33H56N4O4/c1-8-23(4)30(32(41)35-21-25-16-12-13-17-34-25)37-31(40)26(22(2)3)19-28(38)27(18-24-14-10-9-11-15-24)36-29(39)20-33(5,6)7/h12-13,16-17,22-24,26-28,30,38H,8-11,14-15,18-21H2,1-7H3,(H,35,41)(H,36,39)(H,37,40)/t23?,26-,27-,28-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50281642

(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50281642

(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of HIV-2 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50281642

(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50273099
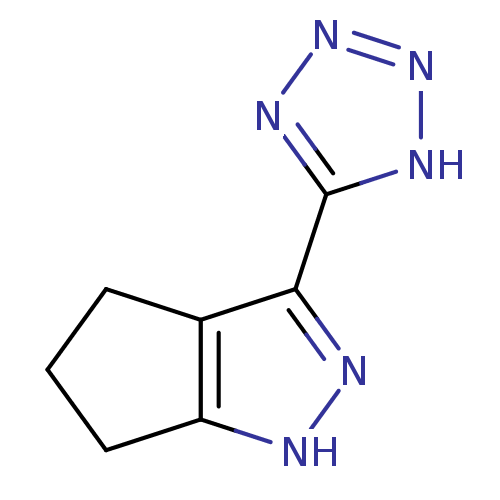
(3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...)Show InChI InChI=1S/C7H8N6/c1-2-4-5(3-1)8-9-6(4)7-10-12-13-11-7/h1-3H2,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281650
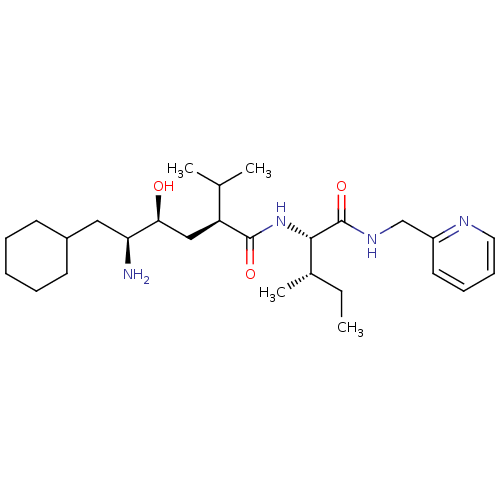
((2S,4S,5S)-5-Amino-6-cyclohexyl-4-hydroxy-2-isopro...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@@H](N)CC1CCCCC1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C27H46N4O3/c1-5-19(4)25(27(34)30-17-21-13-9-10-14-29-21)31-26(33)22(18(2)3)16-24(32)23(28)15-20-11-7-6-8-12-20/h9-10,13-14,18-20,22-25,32H,5-8,11-12,15-17,28H2,1-4H3,(H,30,34)(H,31,33)/t19-,22-,23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281647
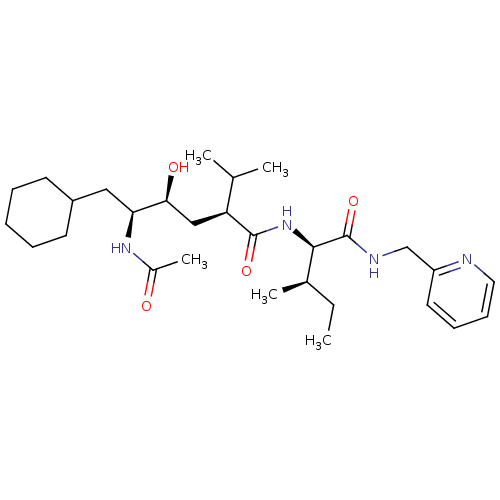
((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(C)=O)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C29H48N4O4/c1-6-20(4)27(29(37)31-18-23-14-10-11-15-30-23)33-28(36)24(19(2)3)17-26(35)25(32-21(5)34)16-22-12-8-7-9-13-22/h10-11,14-15,19-20,22,24-27,35H,6-9,12-13,16-18H2,1-5H3,(H,31,37)(H,32,34)(H,33,36)/t20-,24+,25+,26+,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281649
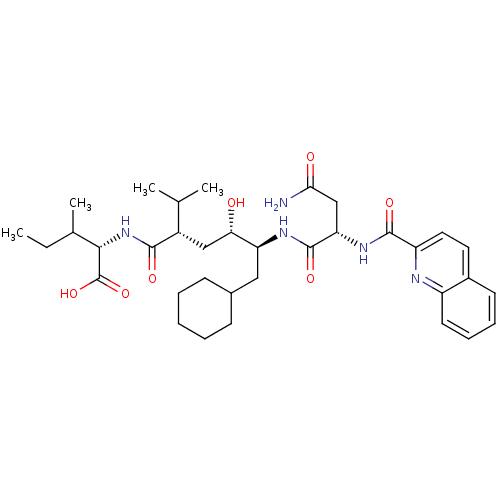
((S)-2-((2S,4S,5S)-5-{(S)-3-Carbamoyl-2-[(quinoline...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(O)=O Show InChI InChI=1S/C35H51N5O7/c1-5-21(4)31(35(46)47)40-32(43)24(20(2)3)18-29(41)27(17-22-11-7-6-8-12-22)38-34(45)28(19-30(36)42)39-33(44)26-16-15-23-13-9-10-14-25(23)37-26/h9-10,13-16,20-22,24,27-29,31,41H,5-8,11-12,17-19H2,1-4H3,(H2,36,42)(H,38,45)(H,39,44)(H,40,43)(H,46,47)/t21?,24-,27-,28-,29-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281642

(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220848
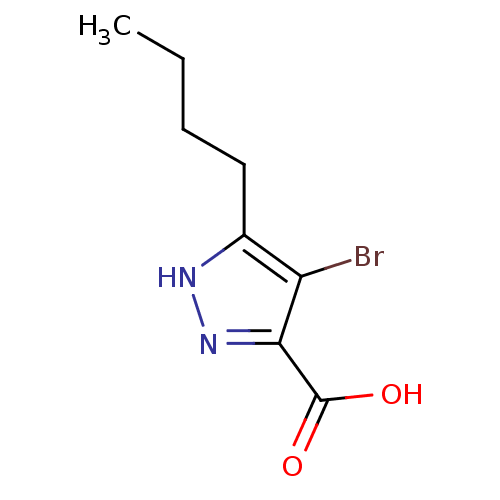
(4-bromo-5-butyl-1H-pyrazole-3-carboxylic acid | CH...)Show InChI InChI=1S/C8H11BrN2O2/c1-2-3-4-5-6(9)7(8(12)13)11-10-5/h2-4H2,1H3,(H,10,11)(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220846
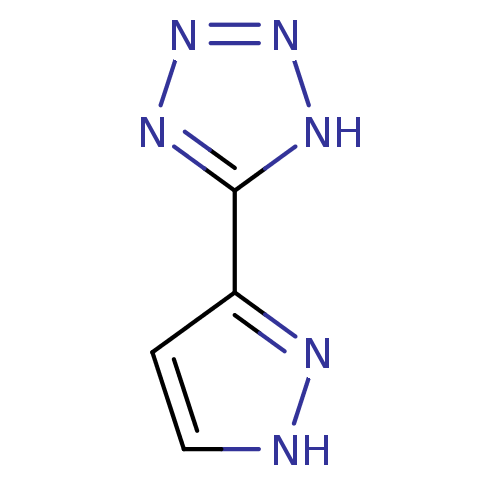
(5-(1H-pyrazol-3-yl)-1H-tetrazole | CHEMBL393063)Show InChI InChI=1S/C4H4N6/c1-2-5-6-3(1)4-7-9-10-8-4/h1-2H,(H,5,6)(H,7,8,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
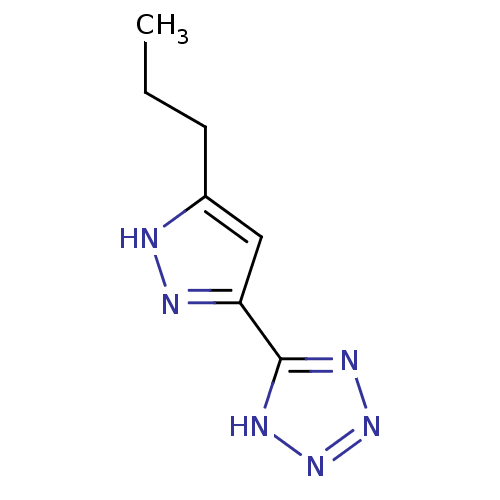
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220833
(5-(5-propyl-1H-pyrazol-3-yl)-1H-tetrazole | CHEMBL...)Show InChI InChI=1S/C7H10N6/c1-2-3-5-4-6(9-8-5)7-10-12-13-11-7/h4H,2-3H2,1H3,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
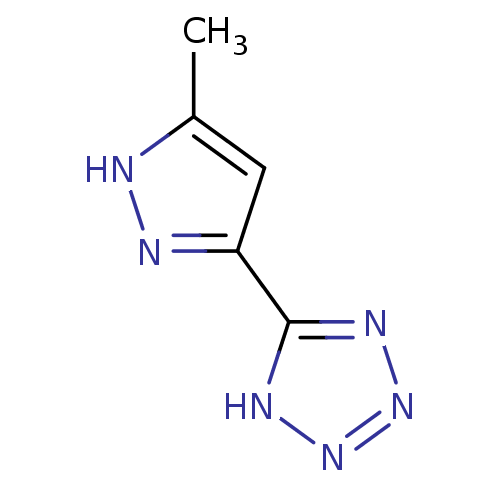
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220840
(5-(5-methyl-1H-pyrazol-3-yl)-1H-tetrazole | CHEMBL...)Show InChI InChI=1S/C5H6N6/c1-3-2-4(7-6-3)5-8-10-11-9-5/h2H,1H3,(H,6,7)(H,8,9,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
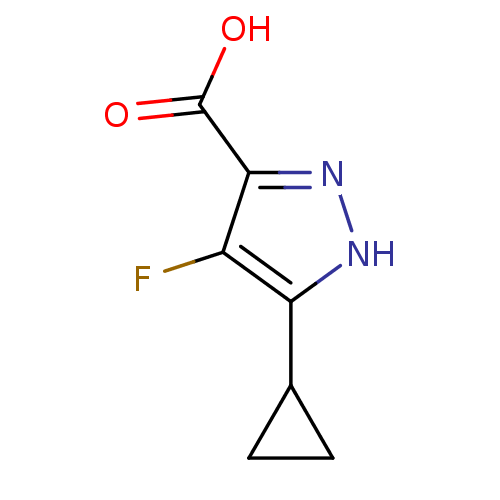
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220854
(5-cyclopropyl-4-fluoro-1H-pyrazole-3-carboxylic ac...)Show InChI InChI=1S/C7H7FN2O2/c8-4-5(3-1-2-3)9-10-6(4)7(11)12/h3H,1-2H2,(H,9,10)(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220847
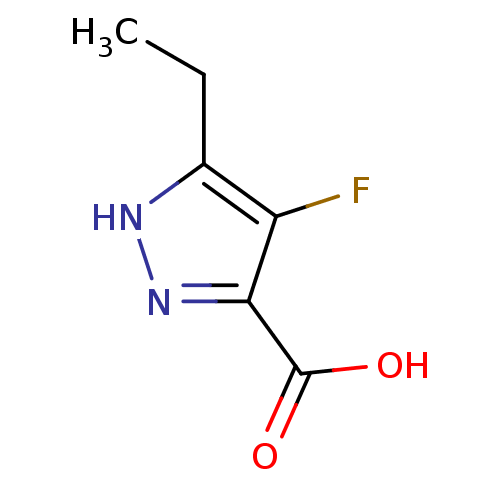
(5-ethyl-4-fluoro-1H-pyrazole-3-carboxylic acid | C...)Show InChI InChI=1S/C6H7FN2O2/c1-2-3-4(7)5(6(10)11)9-8-3/h2H2,1H3,(H,8,9)(H,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220851
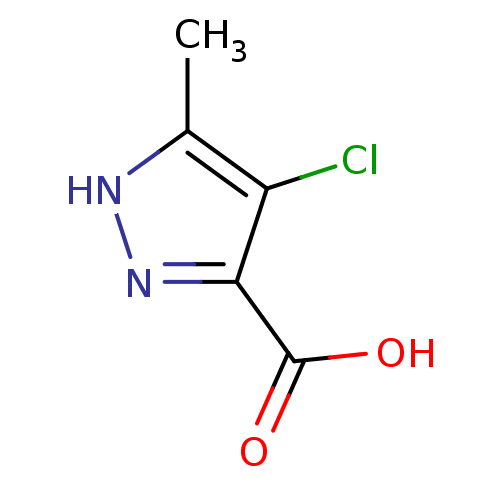
(4-chloro-5-methyl-1H-pyrazole-3-carboxylic acid | ...)Show InChI InChI=1S/C5H5ClN2O2/c1-2-3(6)4(5(9)10)8-7-2/h1H3,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220839
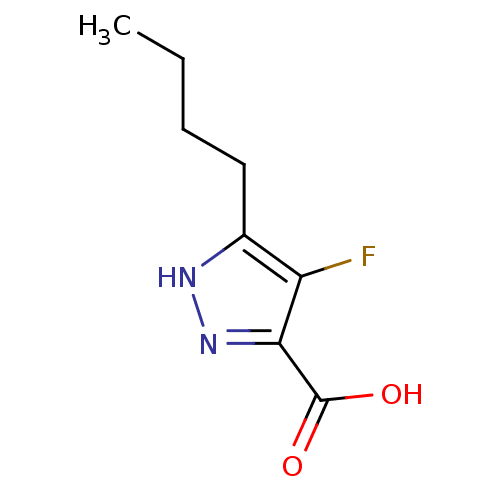
(5-butyl-4-fluoro-1H-pyrazole-3-carboxylic acid | C...)Show InChI InChI=1S/C8H11FN2O2/c1-2-3-4-5-6(9)7(8(12)13)11-10-5/h2-4H2,1H3,(H,10,11)(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220853
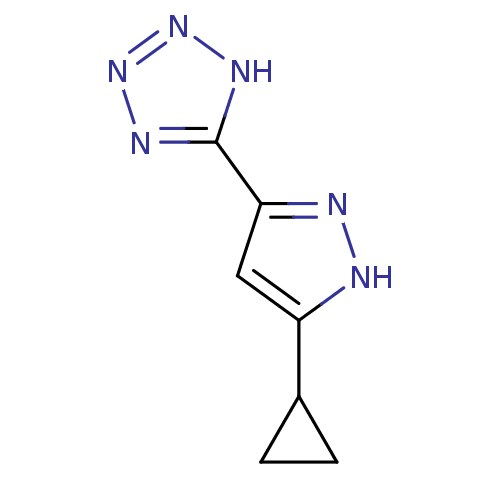
(5-(5-cyclopropyl-1H-pyrazol-3-yl)-1H-tetrazole | C...)Show InChI InChI=1S/C7H8N6/c1-2-4(1)5-3-6(9-8-5)7-10-12-13-11-7/h3-4H,1-2H2,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50220838
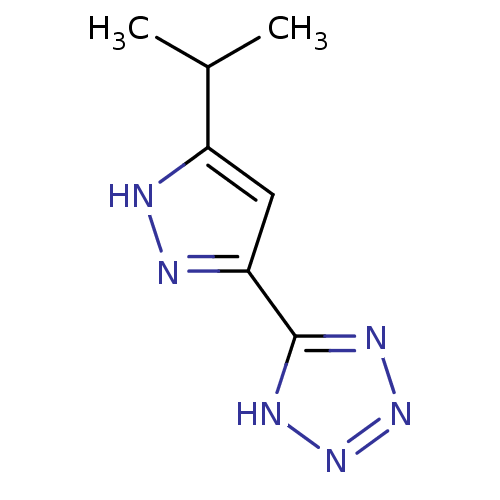
(5-(5-isopropyl-1H-pyrazol-3-yl)-1H-tetrazole | CHE...)Show InChI InChI=1S/C7H10N6/c1-4(2)5-3-6(9-8-5)7-10-12-13-11-7/h3-4H,1-2H3,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50216539
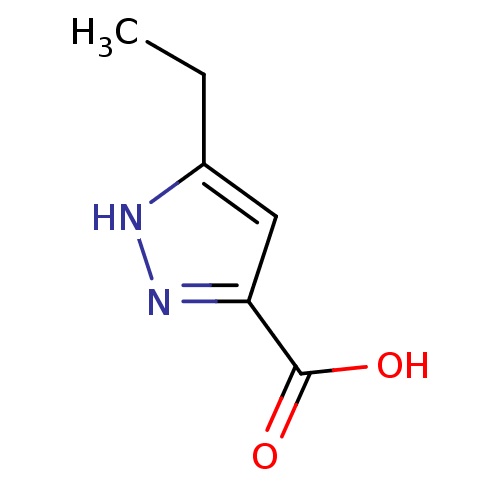
(3-ethyl-1H-pyrazole-5-carboxylic acid | 5-ethyl-1H...)Show InChI InChI=1S/C6H8N2O2/c1-2-4-3-5(6(9)10)8-7-4/h3H,2H2,1H3,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data