

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50581791 (CHEMBL5073221) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV-1 protease assessed as initial inhibition constant by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518599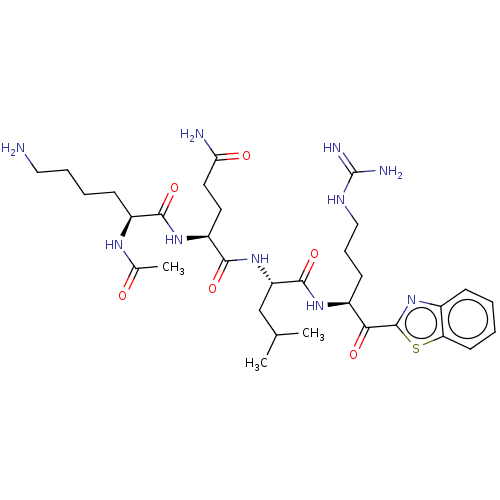 (CHEMBL4454016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518590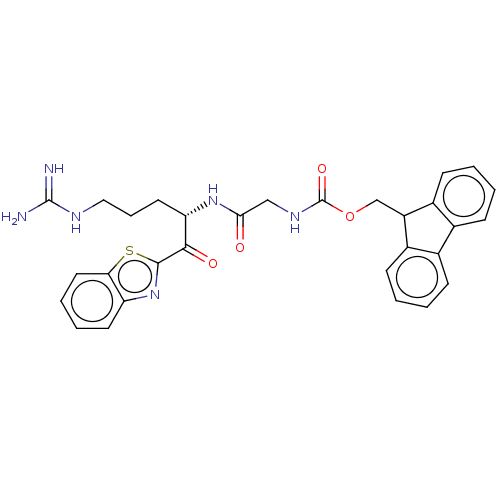 (CHEMBL4548036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518598 (CHEMBL4540950) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032697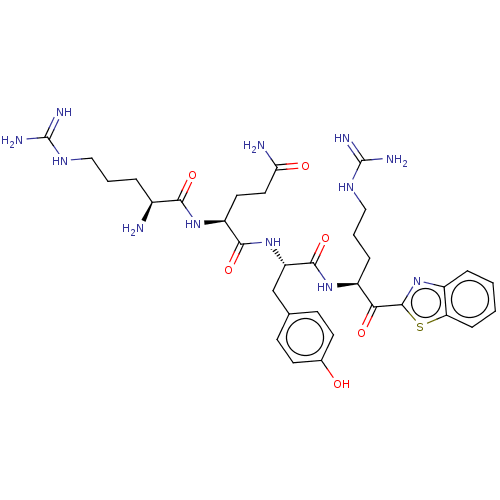 (CHEMBL3354675) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518597 (CHEMBL4469801) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518606 (CHEMBL4562668) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518609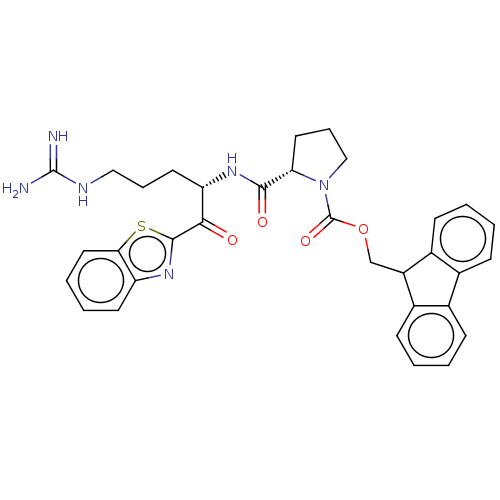 (CHEMBL4571215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518599 (CHEMBL4454016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518605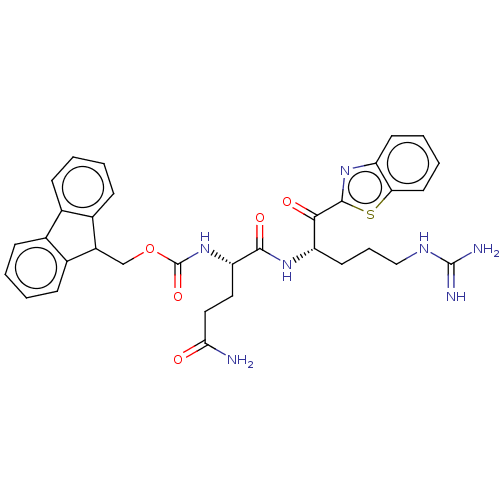 (CHEMBL4567317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50212827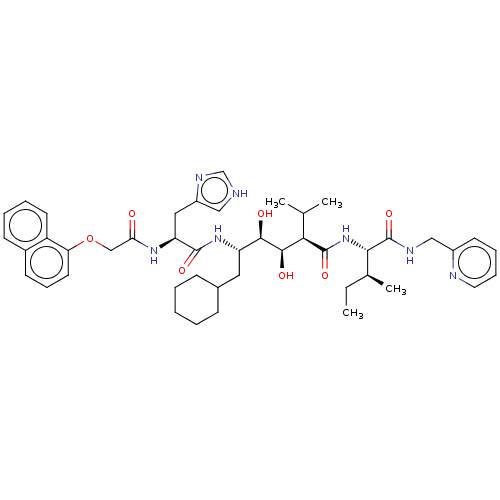 (CHEMBL3350189) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518596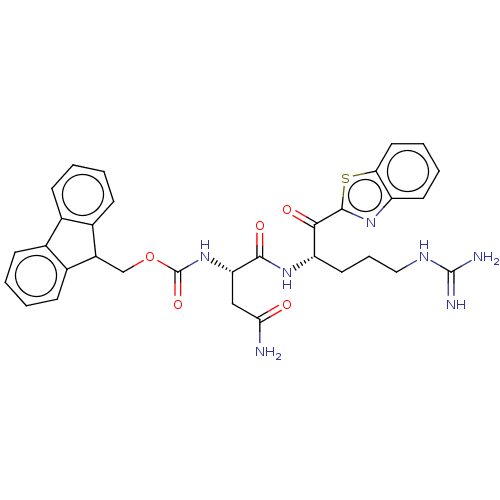 (CHEMBL4463174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50281638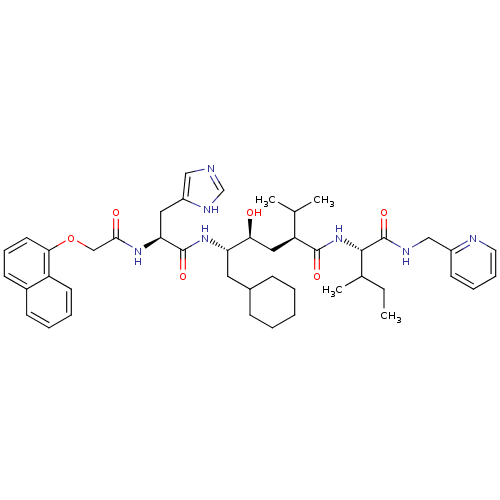 ((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to renin | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50251208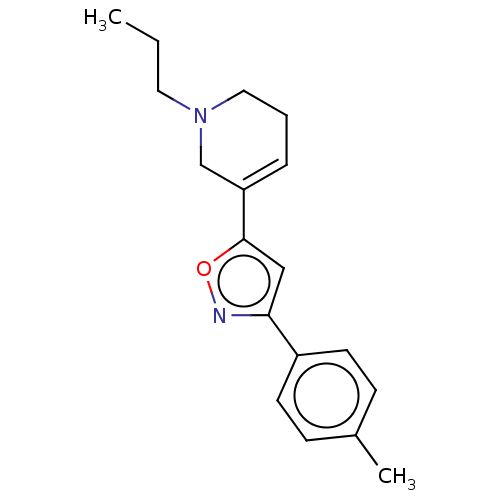 (CHEMBL4088272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50048866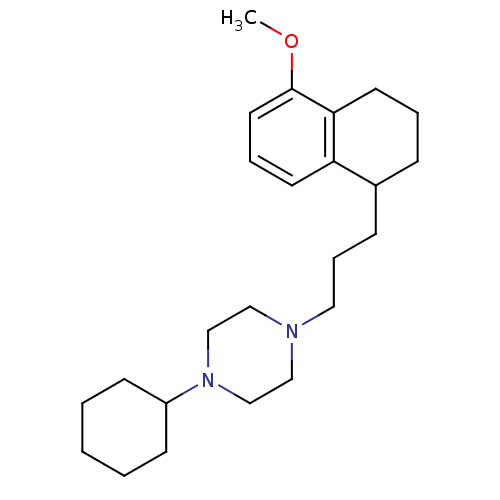 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50212827 (CHEMBL3350189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to renin | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50281636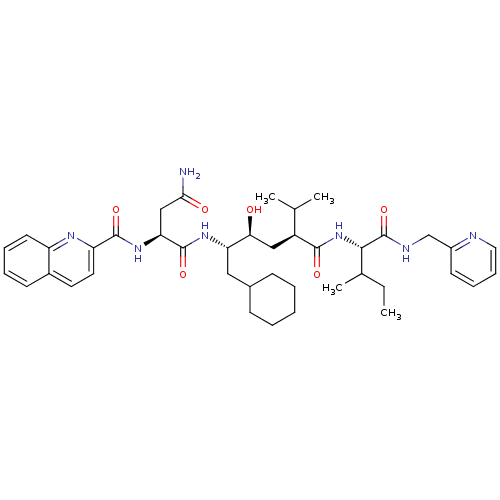 ((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50281636 ((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518598 (CHEMBL4540950) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518607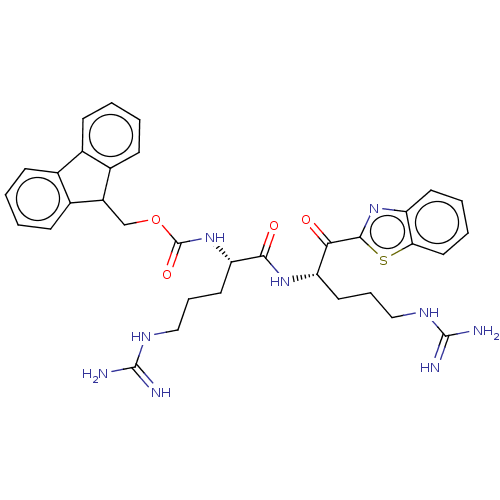 (CHEMBL4562796) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281641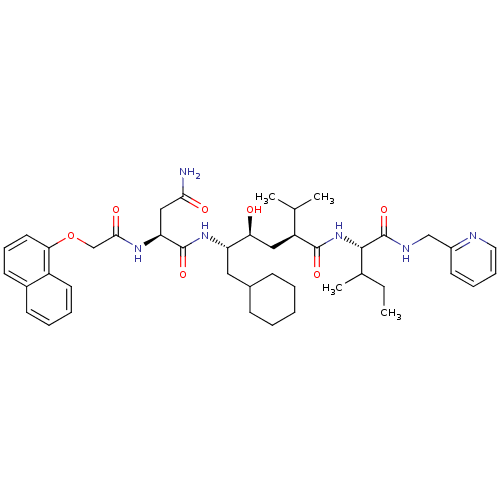 ((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518603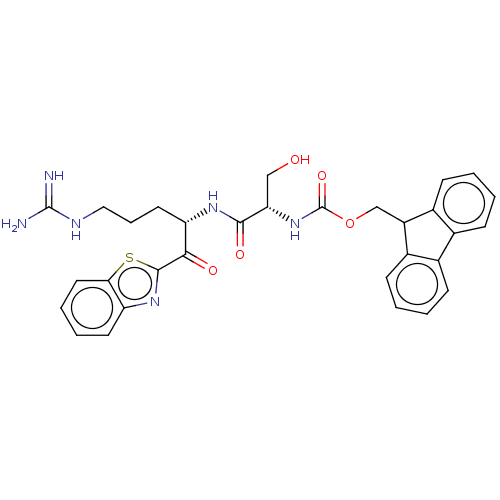 (CHEMBL4472544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281638 ((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518591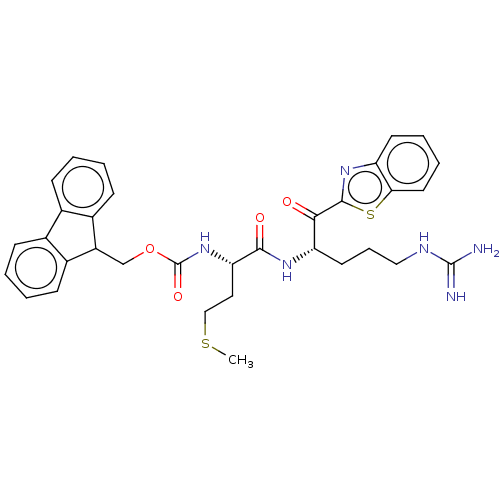 (CHEMBL4552828) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518593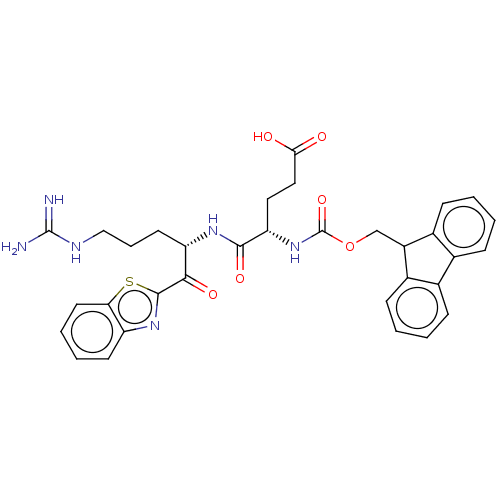 (CHEMBL4467638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281642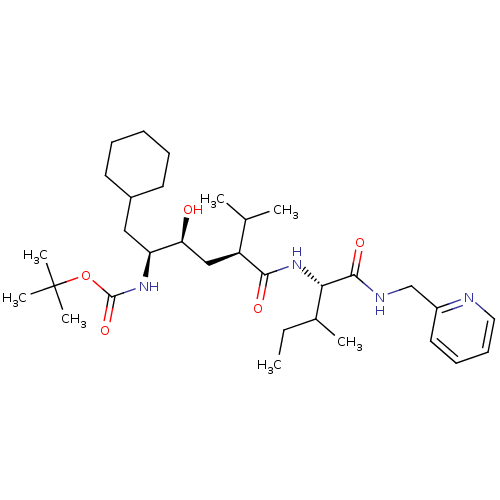 (((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281640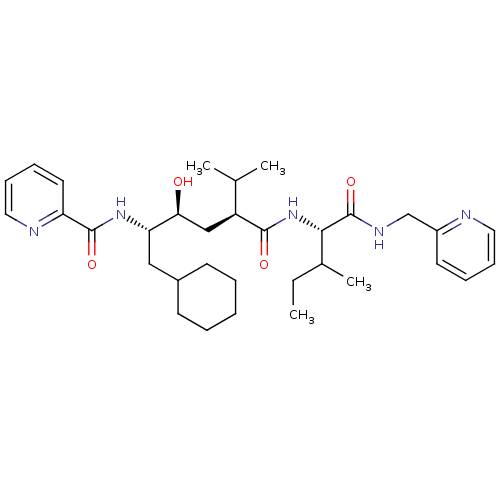 (CHEMBL433729 | Pyridine-2-carboxylic acid ((1S,2S,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281651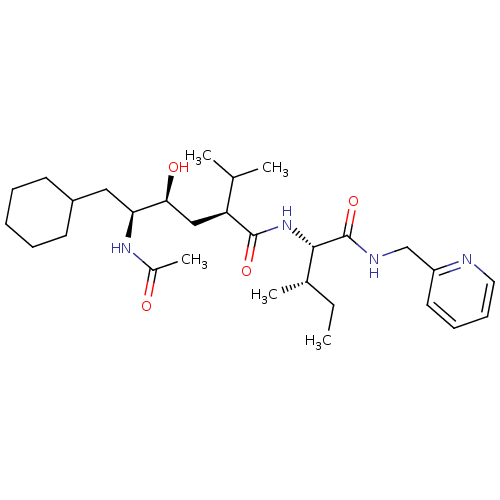 ((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281636 ((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50253157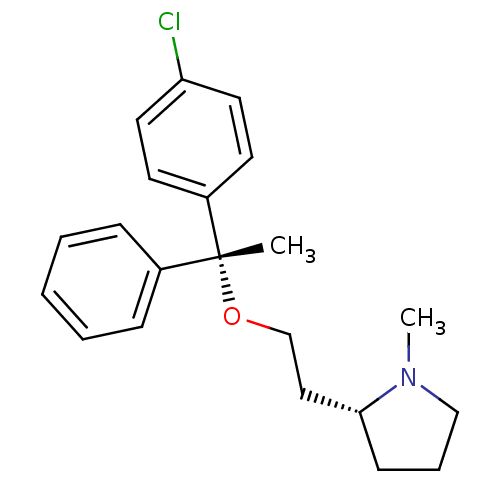 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281648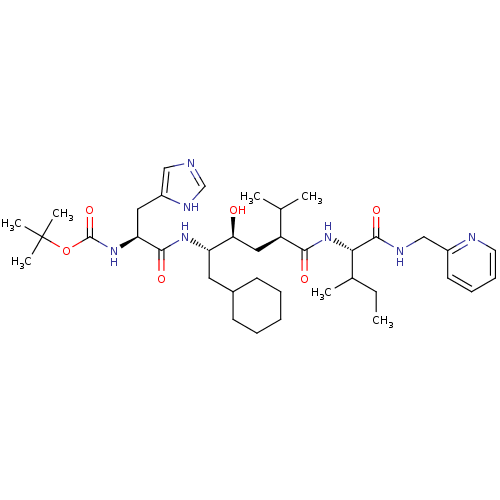 (CHEMBL166640 | [(S)-1-((1S,2S,4S)-1-Cyclohexylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518604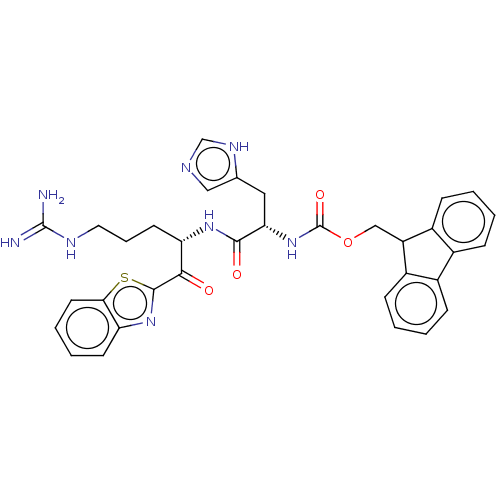 (CHEMBL4551624) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50281636 ((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsin | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281644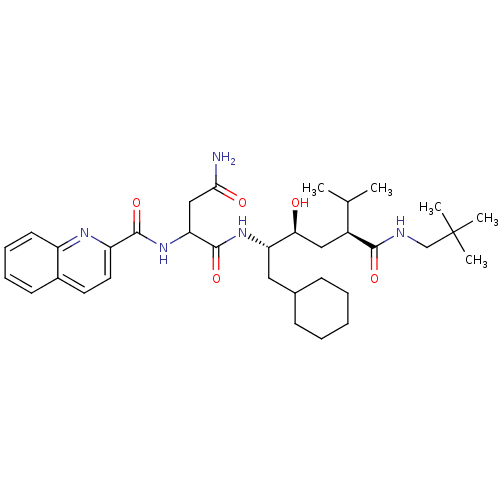 (CHEMBL355867 | N*1*-[(1S,2S,4S)-1-Cyclohexylmethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281643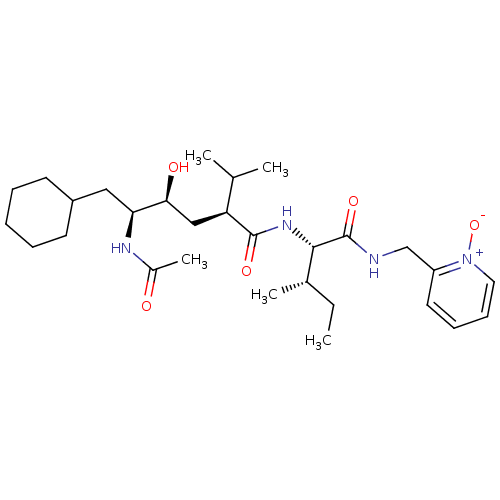 ((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518595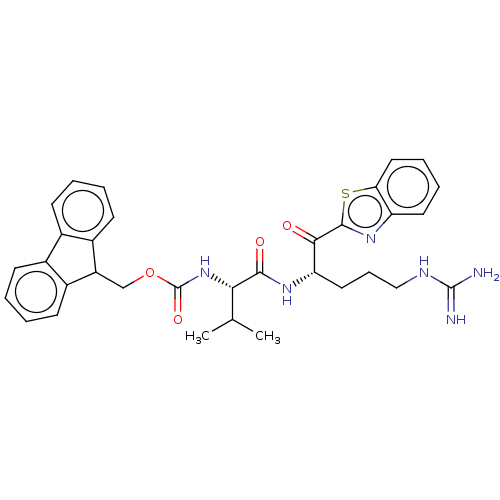 (CHEMBL4472547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518601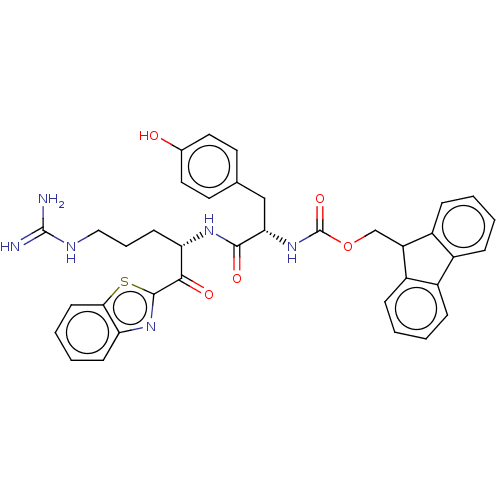 (CHEMBL4460256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281652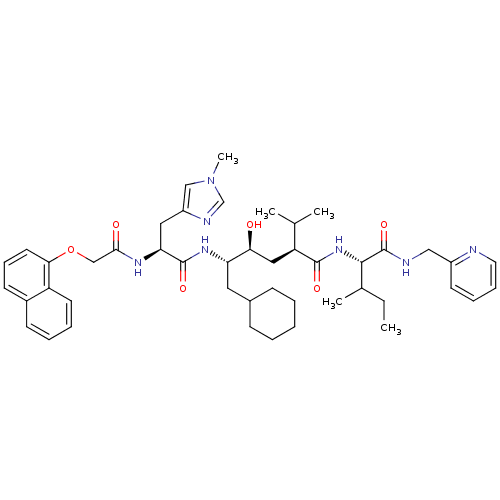 ((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-2-isopropyl-5-{(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518599 (CHEMBL4454016) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50212827 (CHEMBL3350189) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of HIV-2 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518598 (CHEMBL4540950) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281639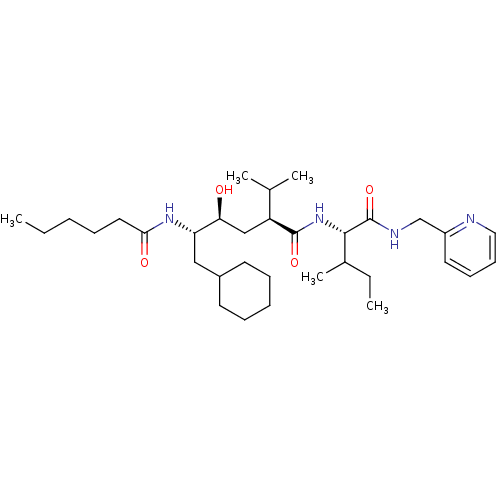 ((2S,4S,5S)-6-Cyclohexyl-5-hexanoylamino-4-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50281638 ((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E | Bioorg Med Chem Lett 3: 819-824 (1993) Article DOI: 10.1016/S0960-894X(00)80673-3 BindingDB Entry DOI: 10.7270/Q2NS0VCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 280 total ) | Next | Last >> |