| Citation |  Brady, SF; Stauffer, KJ; Lumma, WC; Smith, GM; Ramjit, HG; Lewis, SD; Lucas, BJ; Gardell, SJ; Lyle, EA; Appleby, SD; Cook, JJ; Holahan, MA; Stranieri, MT; Lynch, JJ; Lin, JH; Chen, IW; Vastag, K; Naylor-Olsen, AM; Vacca, JP Discovery and development of the novel potent orally active thrombin inhibitor N-(9-hydroxy-9-fluorenecarboxy)prolyl trans-4-aminocyclohexylmethyl amide (L-372,460): coapplication of structure-based design and rapid multiple analogue synthesis on solid support. J Med Chem41:401-6 (1998) [PubMed] Article Brady, SF; Stauffer, KJ; Lumma, WC; Smith, GM; Ramjit, HG; Lewis, SD; Lucas, BJ; Gardell, SJ; Lyle, EA; Appleby, SD; Cook, JJ; Holahan, MA; Stranieri, MT; Lynch, JJ; Lin, JH; Chen, IW; Vastag, K; Naylor-Olsen, AM; Vacca, JP Discovery and development of the novel potent orally active thrombin inhibitor N-(9-hydroxy-9-fluorenecarboxy)prolyl trans-4-aminocyclohexylmethyl amide (L-372,460): coapplication of structure-based design and rapid multiple analogue synthesis on solid support. J Med Chem41:401-6 (1998) [PubMed] Article |
|---|
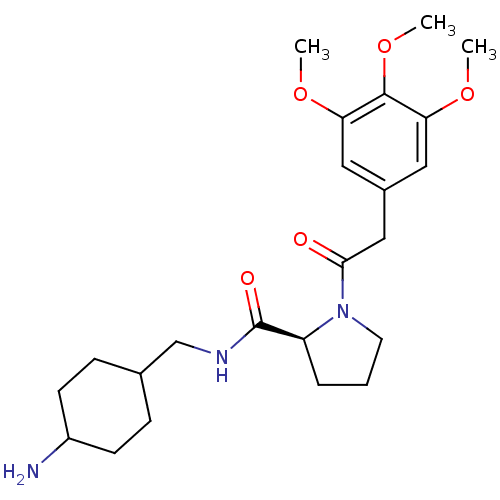
| SMILES | COc1cc(CC(=O)N2CCC[C@H]2C(=O)NCC2CCC(N)CC2)cc(OC)c1OC |wU:12.13,(-.58,-12.31,;.03,-13.72,;1.57,-13.88,;2.47,-12.65,;4,-12.81,;4.91,-11.57,;6.44,-11.73,;7.05,-13.14,;7.35,-10.48,;6.86,-9.01,;8.12,-8.12,;9.36,-9.01,;8.89,-10.48,;9.78,-11.73,;9.15,-13.14,;11.32,-11.57,;11.94,-10.17,;11.53,-8.68,;11.53,-7.14,;10.25,-5.57,;11.44,-4.24,;11.99,-2.81,;11.41,-5.69,;12.72,-7.35,;4.63,-14.21,;3.72,-15.46,;4.35,-16.87,;3.44,-18.1,;2.19,-15.3,;1.28,-16.54,;-.25,-16.38,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Brady, SF; Stauffer, KJ; Lumma, WC; Smith, GM; Ramjit, HG; Lewis, SD; Lucas, BJ; Gardell, SJ; Lyle, EA; Appleby, SD; Cook, JJ; Holahan, MA; Stranieri, MT; Lynch, JJ; Lin, JH; Chen, IW; Vastag, K; Naylor-Olsen, AM; Vacca, JP Discovery and development of the novel potent orally active thrombin inhibitor N-(9-hydroxy-9-fluorenecarboxy)prolyl trans-4-aminocyclohexylmethyl amide (L-372,460): coapplication of structure-based design and rapid multiple analogue synthesis on solid support. J Med Chem41:401-6 (1998) [PubMed] Article
Brady, SF; Stauffer, KJ; Lumma, WC; Smith, GM; Ramjit, HG; Lewis, SD; Lucas, BJ; Gardell, SJ; Lyle, EA; Appleby, SD; Cook, JJ; Holahan, MA; Stranieri, MT; Lynch, JJ; Lin, JH; Chen, IW; Vastag, K; Naylor-Olsen, AM; Vacca, JP Discovery and development of the novel potent orally active thrombin inhibitor N-(9-hydroxy-9-fluorenecarboxy)prolyl trans-4-aminocyclohexylmethyl amide (L-372,460): coapplication of structure-based design and rapid multiple analogue synthesis on solid support. J Med Chem41:401-6 (1998) [PubMed] Article