 Found 91 hits with Last Name = 'appleby' and Initial = 'sd'
Found 91 hits with Last Name = 'appleby' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50056772
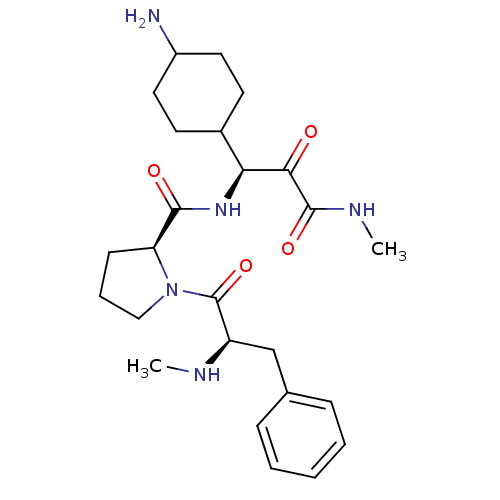
((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C1CCC(N)CC1)C(=O)C(=O)NC |wU:16.18,20.21,wD:2.1,(5.3,-14.61,;6.8,-15.03,;7.89,-13.94,;6.56,-13.17,;6.56,-11.63,;7.89,-10.85,;7.89,-9.32,;6.56,-8.55,;5.21,-9.32,;5.23,-10.86,;9.43,-14.1,;10.06,-15.5,;10.34,-12.86,;9.87,-11.39,;11.11,-10.48,;12.35,-11.39,;11.88,-12.86,;12.79,-14.1,;12.16,-15.5,;14.31,-13.94,;14.94,-12.54,;14.54,-11.04,;15.73,-9.71,;14.4,-8.06,;14.43,-6.61,;15,-5.16,;13.24,-7.94,;14.52,-9.5,;16.43,-12.93,;17.51,-11.84,;16.82,-14.42,;15.73,-15.5,;18.3,-14.82,;19.4,-13.73,)| Show InChI InChI=1S/C25H37N5O4/c1-27-19(15-16-7-4-3-5-8-16)25(34)30-14-6-9-20(30)23(32)29-21(22(31)24(33)28-2)17-10-12-18(26)13-11-17/h3-5,7-8,17-21,27H,6,9-15,26H2,1-2H3,(H,28,33)(H,29,32)/t17?,18?,19-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of the compound against human thrombin was determined |
Bioorg Med Chem Lett 7: 67-72 (1997)
Article DOI: 10.1016/S0960-894X(96)00583-5
BindingDB Entry DOI: 10.7270/Q2639PQM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50454822
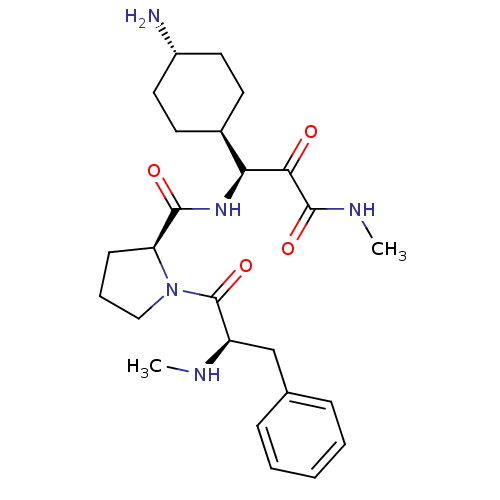
(CHEMBL2062141 | L-370518)Show SMILES [H][C@@](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC)(C(=O)C(=O)NC)[C@@]1([H])CC[C@H](N)CC1 |wU:1.0,wD:12.13,5.4,32.34,28.30,(9.54,-15.25,;8.45,-16.34,;7.42,-17.49,;5.92,-17.17,;5.44,-15.7,;4.89,-18.31,;5.21,-19.82,;3.87,-20.59,;2.73,-19.56,;3.35,-18.15,;2.58,-16.82,;3.35,-15.48,;1.04,-16.82,;.27,-15.48,;1.04,-14.15,;2.58,-14.15,;3.35,-12.82,;2.58,-11.48,;1.04,-11.48,;.27,-12.82,;.27,-18.15,;-1.27,-18.15,;7.98,-14.88,;6.47,-14.56,;9.01,-13.73,;10.51,-14.05,;8.53,-12.27,;9.56,-11.12,;9.96,-16.66,;8.87,-17.75,;10.99,-15.52,;12.5,-15.84,;12.97,-17.3,;14.48,-17.62,;11.94,-18.45,;10.44,-18.13,)| Show InChI InChI=1S/C25H37N5O4/c1-27-19(15-16-7-4-3-5-8-16)25(34)30-14-6-9-20(30)23(32)29-21(22(31)24(33)28-2)17-10-12-18(26)13-11-17/h3-5,7-8,17-21,27H,6,9-15,26H2,1-2H3,(H,28,33)(H,29,32)/t17-,18-,19-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057828

((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.37,-6.36,;9.7,-5.59,;9.7,-4.05,;11.04,-3.28,;12.58,-3.28,;13.35,-1.94,;12.56,-.61,;11.02,-.62,;10.27,-1.96,;8.35,-3.28,;8.35,-1.74,;7.03,-.97,;5.69,-1.74,;5.69,-3.28,;7.03,-4.05,;11.05,-6.36,;11.05,-7.9,;12.36,-5.59,;12.52,-4.05,;14.03,-3.73,;14.8,-5.05,;13.77,-6.2,;14.1,-7.71,;12.96,-8.74,;15.57,-8.18,;16.72,-7.15,;18.17,-7.61,;18.09,-8.95,;19.1,-10.37,;17.98,-11.46,;19.06,-12.54,;18.09,-10.21,;17.07,-8.71,)| Show InChI InChI=1S/C27H36N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,19,22-25H,7,12-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057828

((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.37,-6.36,;9.7,-5.59,;9.7,-4.05,;11.04,-3.28,;12.58,-3.28,;13.35,-1.94,;12.56,-.61,;11.02,-.62,;10.27,-1.96,;8.35,-3.28,;8.35,-1.74,;7.03,-.97,;5.69,-1.74,;5.69,-3.28,;7.03,-4.05,;11.05,-6.36,;11.05,-7.9,;12.36,-5.59,;12.52,-4.05,;14.03,-3.73,;14.8,-5.05,;13.77,-6.2,;14.1,-7.71,;12.96,-8.74,;15.57,-8.18,;16.72,-7.15,;18.17,-7.61,;18.09,-8.95,;19.1,-10.37,;17.98,-11.46,;19.06,-12.54,;18.09,-10.21,;17.07,-8.71,)| Show InChI InChI=1S/C27H36N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,19,22-25H,7,12-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057827
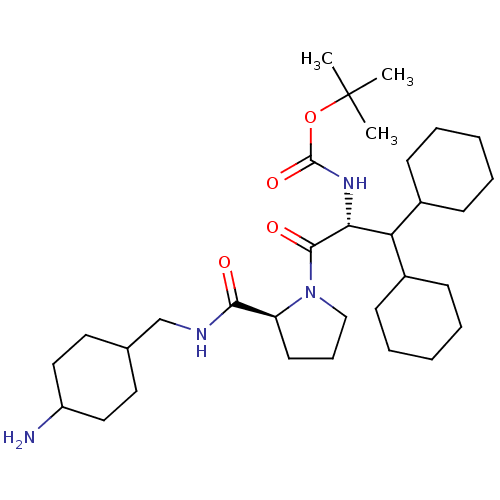
(((R)-2-{(S)-2-[(4-Amino-cyclohexylmethyl)-carbamoy...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:28.31,wD:8.7,(4.89,-7.9,;5.69,-6.58,;4.94,-5.24,;6.75,-7.68,;7.76,-6.72,;8.02,-5.27,;7.26,-3.92,;9.56,-5.29,;10.89,-4.53,;10.89,-2.99,;9.56,-2.21,;9.56,-.67,;8.22,.1,;6.9,-.67,;6.9,-2.21,;8.22,-2.98,;12.23,-2.22,;13.55,-2.99,;14.9,-2.22,;14.9,-.68,;13.57,.09,;12.22,-.68,;12.23,-5.3,;12.23,-6.84,;13.71,-4.89,;14.26,-3.44,;15.79,-3.51,;16.21,-5,;14.92,-5.85,;14.85,-7.39,;13.49,-8.1,;16.15,-8.21,;17.52,-7.51,;18.81,-8.32,;17.75,-9.44,;18.84,-10.92,;18.78,-12.17,;19.86,-13.25,;19.86,-11.05,;18.78,-9.66,)| Show InChI InChI=1S/C32H56N4O4/c1-32(2,3)40-31(39)35-28(27(23-11-6-4-7-12-23)24-13-8-5-9-14-24)30(38)36-20-10-15-26(36)29(37)34-21-22-16-18-25(33)19-17-22/h22-28H,4-21,33H2,1-3H3,(H,34,37)(H,35,39)/t22?,25?,26-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060708
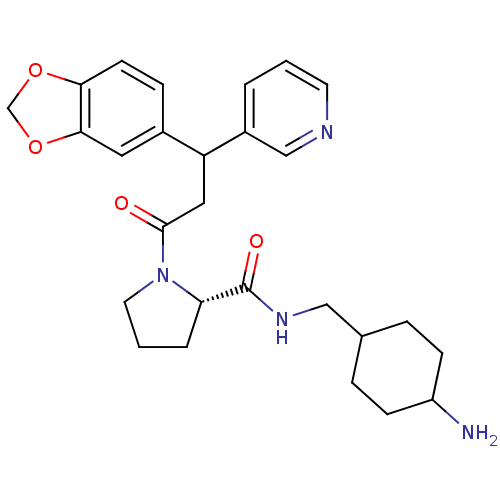
((S)-1-(3-Benzo[1,3]dioxol-5-yl-3-pyridin-3-yl-prop...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2cccnc2)c2ccc3OCOc3c2)CC1 |wU:9.8,(3.95,-11.95,;2.99,-10.74,;1.67,-9.97,;2.29,-8.27,;1.9,-7.1,;.49,-6.47,;.33,-4.95,;-1.06,-4.31,;-2.31,-5.22,;-1.22,-2.77,;-.09,-1.75,;-.71,-.35,;-2.24,-.51,;-2.56,-2,;-3.97,-2.64,;-4.13,-4.16,;-5.21,-1.7,;-5.23,-.17,;-6.56,.6,;-6.56,2.15,;-7.87,2.92,;-9.22,2.15,;-9.21,.6,;-7.87,-.16,;-3.89,.6,;-2.54,-.17,;-1.21,.6,;-1.21,2.15,;-.06,3.2,;-.71,4.62,;-2.25,4.43,;-2.56,2.91,;-3.89,2.14,;3.22,-7.87,;2.58,-9.48,)| Show InChI InChI=1S/C27H34N4O4/c28-21-8-5-18(6-9-21)15-30-27(33)23-4-2-12-31(23)26(32)14-22(20-3-1-11-29-16-20)19-7-10-24-25(13-19)35-17-34-24/h1,3,7,10-11,13,16,18,21-23H,2,4-6,8-9,12,14-15,17,28H2,(H,30,33)/t18?,21?,22?,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50164268
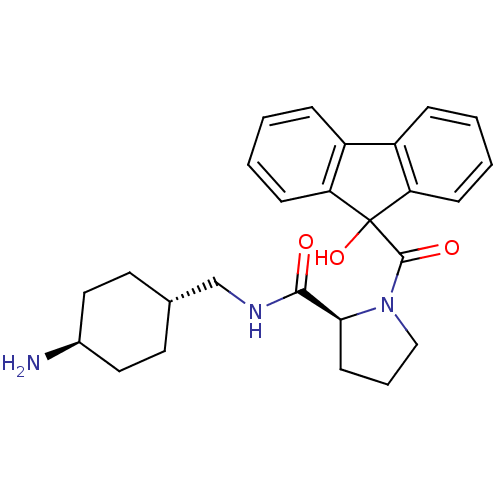
((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...)Show SMILES N[C@H]1CC[C@H](CNC(=O)[C@@H]2CCCN2C(=O)C2(O)c3ccccc3-c3ccccc23)CC1 |wU:9.8,4.4,wD:1.0,(9.04,3.54,;7.74,2.72,;6.37,3.44,;5.07,2.63,;5.13,1.09,;3.83,.27,;2.46,.99,;1.17,.16,;1.22,-1.38,;-.2,.88,;.28,2.35,;-.97,3.26,;-2.21,2.35,;-1.74,.88,;-2.49,-.45,;-1.72,-1.78,;-4.03,-.45,;-3.28,-1.78,;-4.41,1.06,;-3.65,2.42,;-4.44,3.73,;-5.98,3.71,;-6.74,2.35,;-5.96,1.04,;-6.48,-.54,;-7.82,-1.31,;-7.85,-2.85,;-6.52,-3.64,;-5.17,-2.88,;-5.15,-1.34,;6.49,.37,;7.79,1.18,)| Show InChI InChI=1S/C26H31N3O3/c27-18-13-11-17(12-14-18)16-28-24(30)23-10-5-15-29(23)25(31)26(32)21-8-3-1-6-19(21)20-7-2-4-9-22(20)26/h1-4,6-9,17-18,23,32H,5,10-16,27H2,(H,28,30)/t17-,18-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060705
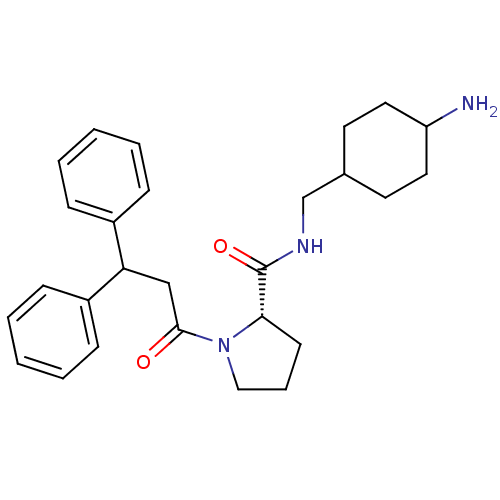
((S)-1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccccc2)CC1 |wU:9.8,(18.93,-14,;17.97,-12.81,;16.65,-12.04,;17.27,-10.34,;16.88,-9.19,;15.48,-8.55,;15.31,-7.01,;13.92,-6.4,;12.67,-7.29,;13.76,-4.86,;14.9,-3.83,;14.28,-2.42,;12.75,-2.58,;12.43,-4.09,;11.02,-4.7,;10.86,-6.24,;9.79,-3.8,;9.76,-2.26,;8.44,-1.49,;7.09,-2.26,;5.78,-1.49,;5.78,.05,;7.09,.82,;8.44,.05,;11.1,-1.49,;12.43,-2.26,;13.76,-1.49,;13.76,.05,;12.42,.82,;11.08,.05,;18.2,-9.96,;17.56,-11.56,)| Show InChI InChI=1S/C27H35N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-6,8-11,20,23-25H,7,12-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50062634

((S)-1-[3-(4-Hydroxy-phenyl)-2-phenyl-propionyl]-py...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)C(Cc2ccc(O)cc2)c2ccccc2)CC1 |wU:9.8,(13.42,-3.24,;12.86,-4.67,;11.67,-6,;12.95,-7.56,;12.95,-9.1,;13.36,-10.6,;12.74,-11.99,;11.2,-12.16,;10.57,-13.57,;10.31,-10.92,;10.78,-9.45,;9.54,-8.54,;8.29,-9.45,;8.77,-10.92,;7.86,-12.16,;8.47,-13.57,;6.33,-11.99,;5.7,-10.6,;4.16,-10.43,;3.27,-11.68,;1.73,-11.52,;1.1,-10.11,;-.42,-9.94,;2.01,-8.87,;3.54,-9.03,;5.42,-13.25,;3.89,-13.08,;2.99,-14.33,;3.61,-15.73,;5.14,-15.89,;6.05,-14.65,;14.14,-7.77,;12.83,-6.12,)| Show InChI InChI=1S/C27H35N3O3/c28-22-12-8-20(9-13-22)18-29-26(32)25-7-4-16-30(25)27(33)24(21-5-2-1-3-6-21)17-19-10-14-23(31)15-11-19/h1-3,5-6,10-11,14-15,20,22,24-25,31H,4,7-9,12-13,16-18,28H2,(H,29,32)/t20?,22?,24?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50062623
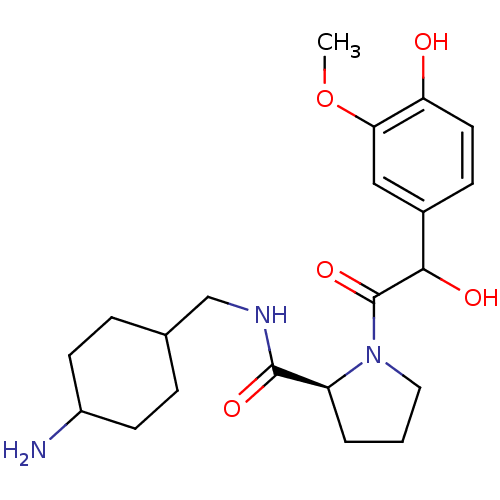
((S)-1-[2-Hydroxy-2-(4-hydroxy-3-methoxy-phenyl)-ac...)Show SMILES COc1cc(ccc1O)C(O)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:17.19,(10.89,-2.92,;9.36,-2.86,;8.54,-4.14,;9.45,-5.36,;8.84,-6.78,;7.33,-6.96,;6.4,-5.72,;7.03,-4.3,;6.1,-3.05,;9.77,-8.01,;8.86,-9.24,;11.28,-8.17,;11.91,-9.57,;12.19,-6.93,;11.72,-5.47,;12.95,-4.57,;14.21,-5.47,;13.72,-6.93,;14.63,-8.17,;14,-9.57,;16.16,-8.01,;16.77,-6.61,;16.37,-5.12,;17.56,-3.8,;16.26,-2.15,;16.28,-.71,;16.84,.74,;15.09,-2.03,;16.37,-3.59,)| Show InChI InChI=1S/C21H31N3O5/c1-29-18-11-14(6-9-17(18)25)19(26)21(28)24-10-2-3-16(24)20(27)23-12-13-4-7-15(22)8-5-13/h6,9,11,13,15-16,19,25-26H,2-5,7-8,10,12,22H2,1H3,(H,23,27)/t13?,15?,16-,19?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50062628
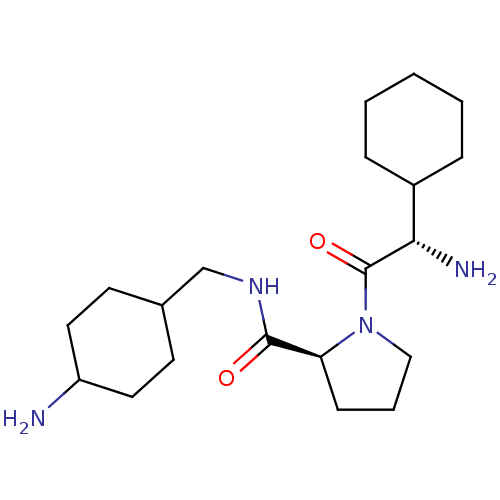
((S)-1-((S)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...)Show SMILES N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:14.16,wD:1.0,(3.03,-10.18,;3.66,-11.59,;2.76,-12.82,;1.22,-12.66,;.33,-13.91,;.94,-15.32,;2.48,-15.48,;3.39,-14.24,;5.18,-11.75,;5.81,-13.15,;6.09,-10.51,;5.62,-9.04,;6.86,-8.14,;8.11,-9.04,;7.63,-10.51,;8.54,-11.75,;7.91,-13.15,;10.07,-11.59,;10.68,-10.19,;10.27,-8.7,;10.27,-7.16,;8.99,-5.6,;10.18,-4.27,;10.75,-2.83,;10.16,-5.72,;11.47,-7.37,)| Show InChI InChI=1S/C20H36N4O2/c21-16-10-8-14(9-11-16)13-23-19(25)17-7-4-12-24(17)20(26)18(22)15-5-2-1-3-6-15/h14-18H,1-13,21-22H2,(H,23,25)/t14?,16?,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060704
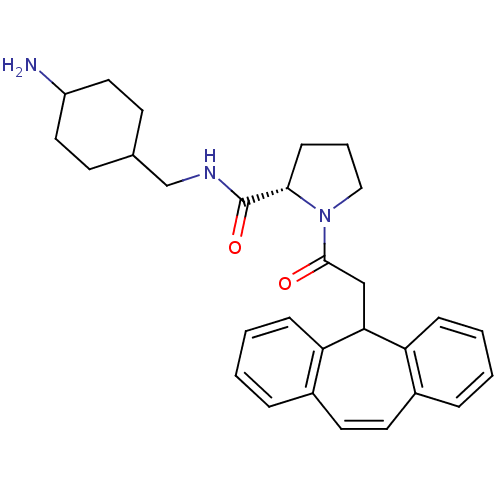
((S)-1-(2-5H-Dibenzo[a,d]cyclohepten-5-yl-acetyl)-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC2c3ccccc3C=Cc3ccccc23)CC1 |wU:9.8,c:26,(10.27,-10.54,;9.32,-9.36,;8.93,-8.11,;9.57,-6.52,;8.23,-5.75,;6.86,-5.12,;6.7,-3.58,;5.27,-2.98,;4.05,-3.87,;5.12,-1.45,;6.29,-.43,;5.65,.98,;4.15,.82,;3.83,-.68,;2.42,-1.29,;2.27,-2.82,;1.18,-.39,;1.18,1.14,;-.35,1.17,;-.93,-.27,;-2.46,-.49,;-3.41,.72,;-2.84,2.16,;-1.31,2.38,;-.96,3.88,;.45,4.52,;1.82,3.82,;2.94,4.87,;4.4,4.42,;4.75,2.92,;3.61,1.87,;2.14,2.32,;8.65,-6.9,;8.01,-8.59,)| Show InChI InChI=1S/C29H35N3O2/c30-23-15-11-20(12-16-23)19-31-29(34)27-10-5-17-32(27)28(33)18-26-24-8-3-1-6-21(24)13-14-22-7-2-4-9-25(22)26/h1-4,6-9,13-14,20,23,26-27H,5,10-12,15-19,30H2,(H,31,34)/t20?,23?,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060703
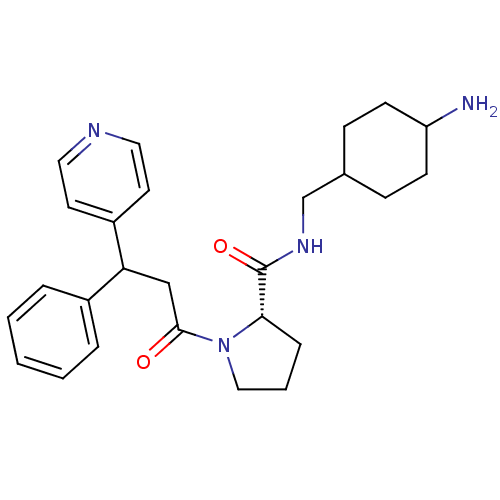
((S)-1-(3-Phenyl-3-pyridin-4-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccncc2)CC1 |wU:9.8,(4.43,-12.54,;3.47,-11.35,;3.06,-10.09,;3.71,-8.48,;2.38,-7.71,;.97,-7.08,;.81,-5.54,;-.58,-4.92,;-1.83,-5.83,;-.74,-3.38,;.4,-2.36,;-.22,-.94,;-1.76,-1.1,;-2.08,-2.61,;-3.49,-3.22,;-3.65,-4.77,;-4.75,-2.32,;-4.75,-.78,;-3.41,-.01,;-2.06,-.78,;-.73,-.01,;-.73,1.53,;-2.08,2.3,;-3.41,1.53,;-6.07,-.01,;-6.07,1.54,;-7.39,2.31,;-8.74,1.54,;-8.73,-.01,;-7.39,-.77,;2.77,-8.88,;2.15,-10.58,)| Show InChI InChI=1S/C26H34N4O2/c27-22-10-8-19(9-11-22)18-29-26(32)24-7-4-16-30(24)25(31)17-23(20-5-2-1-3-6-20)21-12-14-28-15-13-21/h1-3,5-6,12-15,19,22-24H,4,7-11,16-18,27H2,(H,29,32)/t19?,22?,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | 1.55E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50366827
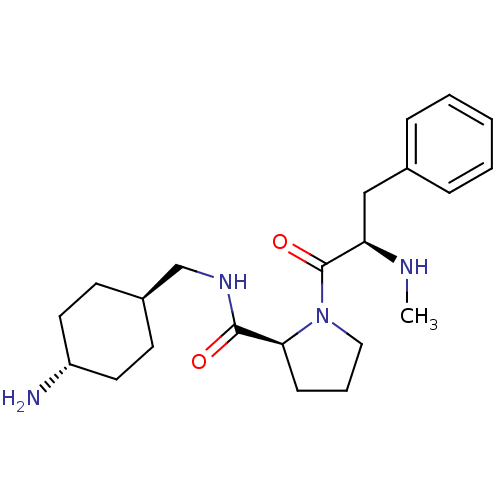
(CHEMBL125181 | L-371912)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC[C@H]1CC[C@H](N)CC1 |wU:16.18,21.22,wD:2.1,24.26,(.43,-4.06,;1.52,-5.16,;3.02,-4.76,;3.15,-3.22,;1.89,-2.34,;2.88,-1.15,;2.33,.28,;.82,.53,;-.16,-.66,;.38,-2.08,;4.42,-5.41,;4.55,-6.95,;5.68,-4.53,;5.69,-2.99,;7.17,-2.53,;8.05,-3.8,;7.12,-5.02,;7.89,-6.35,;7.87,-7.89,;9.38,-5.96,;10.46,-7.04,;11.78,-6.27,;13.32,-6.27,;14.89,-4.99,;16.21,-6.19,;17.54,-5.41,;14.77,-6.16,;13.11,-7.46,)| Show InChI InChI=1S/C22H34N4O2/c1-24-19(14-16-6-3-2-4-7-16)22(28)26-13-5-8-20(26)21(27)25-15-17-9-11-18(23)12-10-17/h2-4,6-7,17-20,24H,5,8-15,23H2,1H3,(H,25,27)/t17-,18-,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50056771

((2S)-N-[(4-aminocyclohexyl)methyl]-1-[(2R)-2-(meth...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC[C@H]1CCC(N)CC1 |wU:16.18,21.22,wD:2.1,(5.43,-11.22,;5.29,-9.68,;6.56,-8.79,;6.43,-7.27,;5.03,-6.59,;3.77,-7.5,;2.37,-6.83,;2.25,-5.29,;3.51,-4.42,;4.91,-5.07,;7.95,-9.44,;8.08,-10.98,;9.21,-8.58,;9.21,-7.04,;10.73,-6.57,;11.61,-7.83,;10.67,-9.07,;11.12,-10.54,;10.07,-11.66,;12.62,-10.89,;13.67,-9.77,;13.22,-8.3,;13.39,-6.83,;12.84,-5.45,;13.92,-4.35,;13.15,-3.02,;13.92,-6.01,;14.44,-7.48,)| Show InChI InChI=1S/C22H34N4O2/c1-24-19(14-16-6-3-2-4-7-16)22(28)26-13-5-8-20(26)21(27)25-15-17-9-11-18(23)12-10-17/h2-4,6-7,17-20,24H,5,8-15,23H2,1H3,(H,25,27)/t17-,18?,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of the compound against human thrombin was determined |
Bioorg Med Chem Lett 7: 67-72 (1997)
Article DOI: 10.1016/S0960-894X(96)00583-5
BindingDB Entry DOI: 10.7270/Q2639PQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50060706
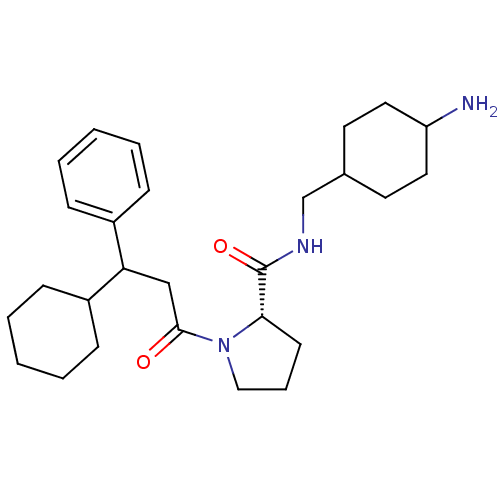
((S)-1-(3-Cyclohexyl-3-phenyl-propionyl)-pyrrolidin...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)c2ccccc2)CC1 |wU:9.8,(12.27,-11.37,;11.3,-10.18,;9.99,-9.41,;10.63,-7.71,;10.21,-6.56,;8.83,-5.91,;8.67,-4.37,;7.26,-3.76,;6.01,-4.66,;7.1,-2.22,;8.26,-1.2,;7.61,.21,;6.11,.05,;5.79,-1.45,;4.37,-2.06,;4.21,-3.6,;3.12,-1.17,;3.12,.37,;4.44,1.14,;4.44,2.68,;5.75,3.45,;7.1,2.68,;7.1,1.14,;5.79,.37,;1.78,1.14,;.43,.37,;-.89,1.14,;-.89,2.68,;.43,3.45,;1.78,2.68,;11.56,-7.33,;10.92,-8.93,)| Show InChI InChI=1S/C27H41N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1,3-4,8-9,20,22-25H,2,5-7,10-19,28H2,(H,29,32)/t20?,23?,24?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060707
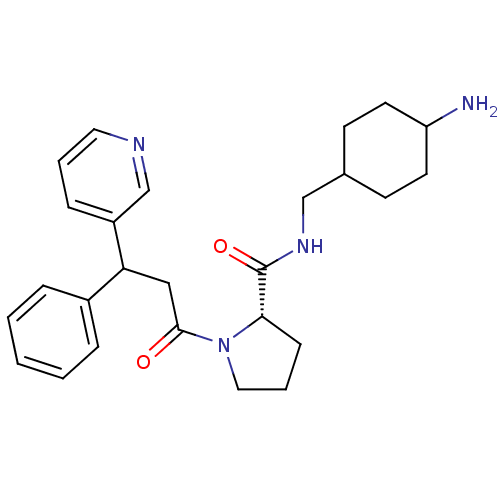
((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2cccnc2)CC1 |wU:9.8,(5.07,-10.18,;4.11,-8.99,;3.7,-7.74,;4.34,-6.14,;3.02,-5.37,;1.62,-4.73,;1.45,-3.19,;.06,-2.58,;-1.19,-3.48,;-.1,-1.04,;1.04,-.01,;.42,1.4,;-1.11,1.24,;-1.43,-.27,;-2.84,-.88,;-3.01,-2.42,;-4.07,.02,;-4.1,1.56,;-2.76,2.33,;-1.41,1.56,;-.09,2.33,;-.09,3.87,;-1.43,4.64,;-2.76,3.87,;-5.42,2.33,;-5.42,3.87,;-6.74,4.64,;-8.09,3.87,;-8.07,2.33,;-6.74,1.56,;3.41,-6.52,;2.79,-8.22,)| Show InChI InChI=1S/C26H34N4O2/c27-22-12-10-19(11-13-22)17-29-26(32)24-9-5-15-30(24)25(31)16-23(20-6-2-1-3-7-20)21-8-4-14-28-18-21/h1-4,6-8,14,18-19,22-24H,5,9-13,15-17,27H2,(H,29,32)/t19?,22?,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060709
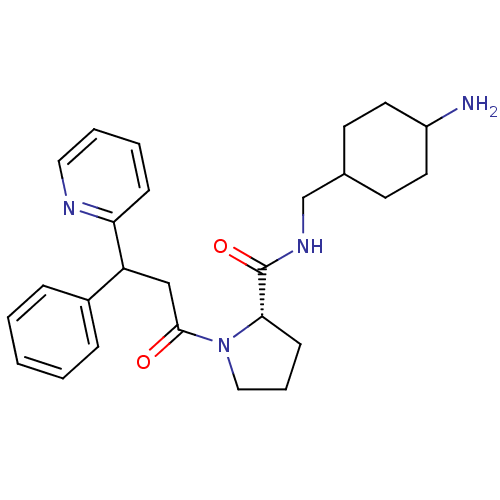
((S)-1-(3-Phenyl-3-pyridin-2-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccccn2)CC1 |wU:9.8,(6.95,-10.61,;5.99,-9.43,;5.61,-8.16,;6.25,-6.56,;4.9,-5.8,;3.53,-5.18,;3.37,-3.64,;1.96,-3.02,;.71,-3.93,;1.8,-1.5,;2.95,-.48,;2.31,.93,;.81,.77,;.49,-.74,;-.92,-1.34,;-1.08,-2.88,;-2.16,-.45,;-2.16,1.09,;-.85,1.85,;.49,1.09,;1.83,1.85,;1.83,3.39,;.49,4.16,;-.85,3.39,;-3.51,1.85,;-3.51,3.41,;-4.82,4.18,;-6.16,3.41,;-6.13,1.85,;-4.82,1.11,;5.32,-6.97,;4.68,-8.66,)| Show InChI InChI=1S/C26H34N4O2/c27-21-13-11-19(12-14-21)18-29-26(32)24-10-6-16-30(24)25(31)17-22(20-7-2-1-3-8-20)23-9-4-5-15-28-23/h1-5,7-9,15,19,21-22,24H,6,10-14,16-18,27H2,(H,29,32)/t19?,21?,22?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060711
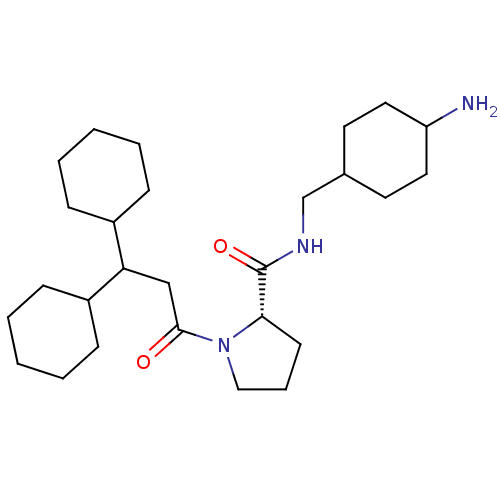
((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060710
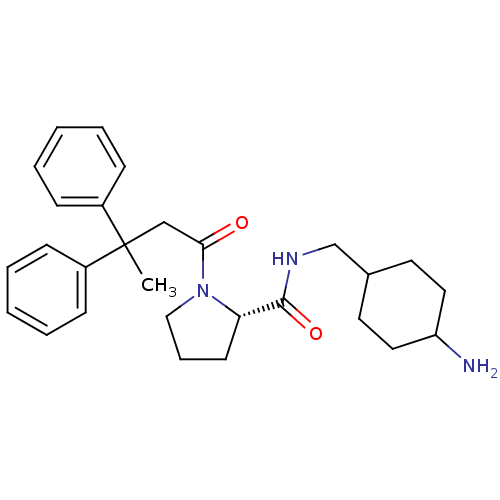
((S)-1-(3,3-Diphenyl-butyryl)-pyrrolidine-2-carboxy...)Show SMILES CC(CC(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1)(c1ccccc1)c1ccccc1 |wU:9.10,(5.27,-.24,;5.29,-1.78,;5.3,-3.32,;6.54,-4.21,;6.38,-5.75,;7.96,-3.6,;8.28,-2.1,;9.8,-1.94,;10.43,-3.35,;9.28,-4.37,;9.44,-5.91,;8.19,-6.81,;10.84,-6.52,;11,-8.06,;12.39,-8.71,;12.8,-9.86,;12.17,-11.56,;13.49,-12.33,;14.45,-13.52,;13.09,-11.08,;13.73,-9.48,;6.62,-1.01,;6.62,.53,;7.96,1.3,;9.3,.53,;9.3,-1.01,;7.97,-1.78,;3.96,-1.01,;3.96,.53,;2.64,1.3,;1.29,.53,;1.32,-1.01,;2.64,-1.78,)| Show InChI InChI=1S/C28H37N3O2/c1-28(22-9-4-2-5-10-22,23-11-6-3-7-12-23)19-26(32)31-18-8-13-25(31)27(33)30-20-21-14-16-24(29)17-15-21/h2-7,9-12,21,24-25H,8,13-20,29H2,1H3,(H,30,33)/t21?,24?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060712

((S)-1-(2-10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC2c3ccccc3CCc3ccccc23)CC1 |wU:9.8,(9.3,-14.48,;8.34,-13.29,;7.94,-12.02,;8.58,-10.42,;7.25,-9.65,;5.86,-9.03,;5.7,-7.49,;4.3,-6.86,;3.05,-7.78,;4.14,-5.35,;5.28,-4.32,;4.65,-2.91,;3.14,-3.07,;2.82,-4.58,;1.41,-5.19,;1.25,-6.73,;.17,-4.29,;.16,-2.75,;1.13,-1.55,;2.61,-2.02,;3.75,-.96,;3.41,.54,;1.93,.99,;.8,-.04,;-.58,.64,;-1.97,,;-2.34,-1.51,;-3.86,-1.73,;-4.45,-3.17,;-3.48,-4.39,;-1.95,-4.16,;-1.38,-2.72,;7.65,-10.83,;7.02,-12.52,)| Show InChI InChI=1S/C29H37N3O2/c30-23-15-11-20(12-16-23)19-31-29(34)27-10-5-17-32(27)28(33)18-26-24-8-3-1-6-21(24)13-14-22-7-2-4-9-25(22)26/h1-4,6-9,20,23,26-27H,5,10-19,30H2,(H,31,34)/t20?,23?,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50062624

((S)-1-(2,3-Dihydro-benzo[1,4]dioxine-2-carbonyl)-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)C2COc3ccccc3O2)CC1 |wU:9.8,(15.05,-.52,;14.47,-1.96,;14.44,-3.4,;15.77,-5.05,;14.58,-6.38,;14.98,-7.87,;14.35,-9.26,;12.84,-9.43,;12.21,-10.82,;11.93,-8.19,;12.4,-6.73,;11.16,-5.82,;9.93,-6.73,;10.4,-8.19,;9.49,-9.43,;10.12,-10.82,;7.95,-9.26,;7.33,-7.85,;5.81,-7.68,;4.91,-8.91,;3.4,-8.75,;2.47,-9.98,;3.09,-11.4,;4.63,-11.57,;5.51,-10.33,;7.05,-10.5,;14.56,-4.85,;13.28,-3.29,)| Show InChI InChI=1S/C21H29N3O4/c22-15-9-7-14(8-10-15)12-23-20(25)16-4-3-11-24(16)21(26)19-13-27-17-5-1-2-6-18(17)28-19/h1-2,5-6,14-16,19H,3-4,7-13,22H2,(H,23,25)/t14?,15?,16-,19?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50057828

((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.37,-6.36,;9.7,-5.59,;9.7,-4.05,;11.04,-3.28,;12.58,-3.28,;13.35,-1.94,;12.56,-.61,;11.02,-.62,;10.27,-1.96,;8.35,-3.28,;8.35,-1.74,;7.03,-.97,;5.69,-1.74,;5.69,-3.28,;7.03,-4.05,;11.05,-6.36,;11.05,-7.9,;12.36,-5.59,;12.52,-4.05,;14.03,-3.73,;14.8,-5.05,;13.77,-6.2,;14.1,-7.71,;12.96,-8.74,;15.57,-8.18,;16.72,-7.15,;18.17,-7.61,;18.09,-8.95,;19.1,-10.37,;17.98,-11.46,;19.06,-12.54,;18.09,-10.21,;17.07,-8.71,)| Show InChI InChI=1S/C27H36N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,19,22-25H,7,12-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human serine protease (Trypsin)(2143*) |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060705

((S)-1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccccc2)CC1 |wU:9.8,(18.93,-14,;17.97,-12.81,;16.65,-12.04,;17.27,-10.34,;16.88,-9.19,;15.48,-8.55,;15.31,-7.01,;13.92,-6.4,;12.67,-7.29,;13.76,-4.86,;14.9,-3.83,;14.28,-2.42,;12.75,-2.58,;12.43,-4.09,;11.02,-4.7,;10.86,-6.24,;9.79,-3.8,;9.76,-2.26,;8.44,-1.49,;7.09,-2.26,;5.78,-1.49,;5.78,.05,;7.09,.82,;8.44,.05,;11.1,-1.49,;12.43,-2.26,;13.76,-1.49,;13.76,.05,;12.42,.82,;11.08,.05,;18.2,-9.96,;17.56,-11.56,)| Show InChI InChI=1S/C27H35N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-6,8-11,20,23-25H,7,12-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057829
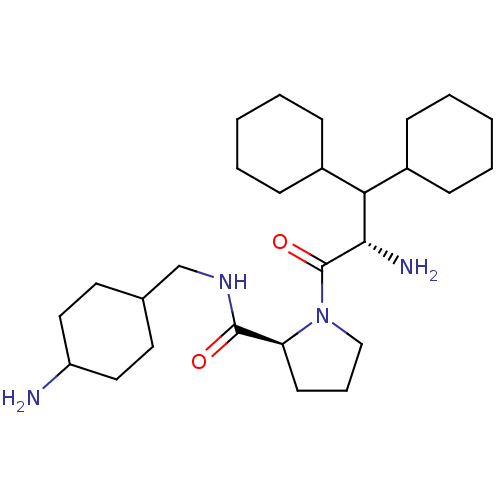
((S)-1-((S)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50164268

((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...)Show SMILES N[C@H]1CC[C@H](CNC(=O)[C@@H]2CCCN2C(=O)C2(O)c3ccccc3-c3ccccc23)CC1 |wU:9.8,4.4,wD:1.0,(9.04,3.54,;7.74,2.72,;6.37,3.44,;5.07,2.63,;5.13,1.09,;3.83,.27,;2.46,.99,;1.17,.16,;1.22,-1.38,;-.2,.88,;.28,2.35,;-.97,3.26,;-2.21,2.35,;-1.74,.88,;-2.49,-.45,;-1.72,-1.78,;-4.03,-.45,;-3.28,-1.78,;-4.41,1.06,;-3.65,2.42,;-4.44,3.73,;-5.98,3.71,;-6.74,2.35,;-5.96,1.04,;-6.48,-.54,;-7.82,-1.31,;-7.85,-2.85,;-6.52,-3.64,;-5.17,-2.88,;-5.15,-1.34,;6.49,.37,;7.79,1.18,)| Show InChI InChI=1S/C26H31N3O3/c27-18-13-11-17(12-14-18)16-28-24(30)23-10-5-15-29(23)25(31)26(32)21-8-3-1-6-19(21)20-7-2-4-9-22(20)26/h1-4,6-9,17-18,23,32H,5,10-16,27H2,(H,28,30)/t17-,18-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards trypsin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50056772

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C1CCC(N)CC1)C(=O)C(=O)NC |wU:16.18,20.21,wD:2.1,(5.3,-14.61,;6.8,-15.03,;7.89,-13.94,;6.56,-13.17,;6.56,-11.63,;7.89,-10.85,;7.89,-9.32,;6.56,-8.55,;5.21,-9.32,;5.23,-10.86,;9.43,-14.1,;10.06,-15.5,;10.34,-12.86,;9.87,-11.39,;11.11,-10.48,;12.35,-11.39,;11.88,-12.86,;12.79,-14.1,;12.16,-15.5,;14.31,-13.94,;14.94,-12.54,;14.54,-11.04,;15.73,-9.71,;14.4,-8.06,;14.43,-6.61,;15,-5.16,;13.24,-7.94,;14.52,-9.5,;16.43,-12.93,;17.51,-11.84,;16.82,-14.42,;15.73,-15.5,;18.3,-14.82,;19.4,-13.73,)| Show InChI InChI=1S/C25H37N5O4/c1-27-19(15-16-7-4-3-5-8-16)25(34)30-14-6-9-20(30)23(32)29-21(22(31)24(33)28-2)17-10-12-18(26)13-11-17/h3-5,7-8,17-21,27H,6,9-15,26H2,1-2H3,(H,28,33)(H,29,32)/t17?,18?,19-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of the compound against bovine trypsin was determined |
Bioorg Med Chem Lett 7: 67-72 (1997)
Article DOI: 10.1016/S0960-894X(96)00583-5
BindingDB Entry DOI: 10.7270/Q2639PQM |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060704

((S)-1-(2-5H-Dibenzo[a,d]cyclohepten-5-yl-acetyl)-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC2c3ccccc3C=Cc3ccccc23)CC1 |wU:9.8,c:26,(10.27,-10.54,;9.32,-9.36,;8.93,-8.11,;9.57,-6.52,;8.23,-5.75,;6.86,-5.12,;6.7,-3.58,;5.27,-2.98,;4.05,-3.87,;5.12,-1.45,;6.29,-.43,;5.65,.98,;4.15,.82,;3.83,-.68,;2.42,-1.29,;2.27,-2.82,;1.18,-.39,;1.18,1.14,;-.35,1.17,;-.93,-.27,;-2.46,-.49,;-3.41,.72,;-2.84,2.16,;-1.31,2.38,;-.96,3.88,;.45,4.52,;1.82,3.82,;2.94,4.87,;4.4,4.42,;4.75,2.92,;3.61,1.87,;2.14,2.32,;8.65,-6.9,;8.01,-8.59,)| Show InChI InChI=1S/C29H35N3O2/c30-23-15-11-20(12-16-23)19-31-29(34)27-10-5-17-32(27)28(33)18-26-24-8-3-1-6-21(24)13-14-22-7-2-4-9-25(22)26/h1-4,6-9,13-14,20,23,26-27H,5,10-12,15-19,30H2,(H,31,34)/t20?,23?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060706

((S)-1-(3-Cyclohexyl-3-phenyl-propionyl)-pyrrolidin...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)c2ccccc2)CC1 |wU:9.8,(12.27,-11.37,;11.3,-10.18,;9.99,-9.41,;10.63,-7.71,;10.21,-6.56,;8.83,-5.91,;8.67,-4.37,;7.26,-3.76,;6.01,-4.66,;7.1,-2.22,;8.26,-1.2,;7.61,.21,;6.11,.05,;5.79,-1.45,;4.37,-2.06,;4.21,-3.6,;3.12,-1.17,;3.12,.37,;4.44,1.14,;4.44,2.68,;5.75,3.45,;7.1,2.68,;7.1,1.14,;5.79,.37,;1.78,1.14,;.43,.37,;-.89,1.14,;-.89,2.68,;.43,3.45,;1.78,2.68,;11.56,-7.33,;10.92,-8.93,)| Show InChI InChI=1S/C27H41N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1,3-4,8-9,20,22-25H,2,5-7,10-19,28H2,(H,29,32)/t20?,23?,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060703

((S)-1-(3-Phenyl-3-pyridin-4-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccncc2)CC1 |wU:9.8,(4.43,-12.54,;3.47,-11.35,;3.06,-10.09,;3.71,-8.48,;2.38,-7.71,;.97,-7.08,;.81,-5.54,;-.58,-4.92,;-1.83,-5.83,;-.74,-3.38,;.4,-2.36,;-.22,-.94,;-1.76,-1.1,;-2.08,-2.61,;-3.49,-3.22,;-3.65,-4.77,;-4.75,-2.32,;-4.75,-.78,;-3.41,-.01,;-2.06,-.78,;-.73,-.01,;-.73,1.53,;-2.08,2.3,;-3.41,1.53,;-6.07,-.01,;-6.07,1.54,;-7.39,2.31,;-8.74,1.54,;-8.73,-.01,;-7.39,-.77,;2.77,-8.88,;2.15,-10.58,)| Show InChI InChI=1S/C26H34N4O2/c27-22-10-8-19(9-11-22)18-29-26(32)24-7-4-16-30(24)25(31)17-23(20-5-2-1-3-6-20)21-12-14-28-15-13-21/h1-3,5-6,12-15,19,22-24H,4,7-11,16-18,27H2,(H,29,32)/t19?,22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060711

((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060708

((S)-1-(3-Benzo[1,3]dioxol-5-yl-3-pyridin-3-yl-prop...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2cccnc2)c2ccc3OCOc3c2)CC1 |wU:9.8,(3.95,-11.95,;2.99,-10.74,;1.67,-9.97,;2.29,-8.27,;1.9,-7.1,;.49,-6.47,;.33,-4.95,;-1.06,-4.31,;-2.31,-5.22,;-1.22,-2.77,;-.09,-1.75,;-.71,-.35,;-2.24,-.51,;-2.56,-2,;-3.97,-2.64,;-4.13,-4.16,;-5.21,-1.7,;-5.23,-.17,;-6.56,.6,;-6.56,2.15,;-7.87,2.92,;-9.22,2.15,;-9.21,.6,;-7.87,-.16,;-3.89,.6,;-2.54,-.17,;-1.21,.6,;-1.21,2.15,;-.06,3.2,;-.71,4.62,;-2.25,4.43,;-2.56,2.91,;-3.89,2.14,;3.22,-7.87,;2.58,-9.48,)| Show InChI InChI=1S/C27H34N4O4/c28-21-8-5-18(6-9-21)15-30-27(33)23-4-2-12-31(23)26(32)14-22(20-3-1-11-29-16-20)19-7-10-24-25(13-19)35-17-34-24/h1,3,7,10-11,13,16,18,21-23H,2,4-6,8-9,12,14-15,17,28H2,(H,30,33)/t18?,21?,22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060707

((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2cccnc2)CC1 |wU:9.8,(5.07,-10.18,;4.11,-8.99,;3.7,-7.74,;4.34,-6.14,;3.02,-5.37,;1.62,-4.73,;1.45,-3.19,;.06,-2.58,;-1.19,-3.48,;-.1,-1.04,;1.04,-.01,;.42,1.4,;-1.11,1.24,;-1.43,-.27,;-2.84,-.88,;-3.01,-2.42,;-4.07,.02,;-4.1,1.56,;-2.76,2.33,;-1.41,1.56,;-.09,2.33,;-.09,3.87,;-1.43,4.64,;-2.76,3.87,;-5.42,2.33,;-5.42,3.87,;-6.74,4.64,;-8.09,3.87,;-8.07,2.33,;-6.74,1.56,;3.41,-6.52,;2.79,-8.22,)| Show InChI InChI=1S/C26H34N4O2/c27-22-12-10-19(11-13-22)17-29-26(32)24-9-5-15-30(24)25(31)16-23(20-6-2-1-3-7-20)21-8-4-14-28-18-21/h1-4,6-8,14,18-19,22-24H,5,9-13,15-17,27H2,(H,29,32)/t19?,22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057830
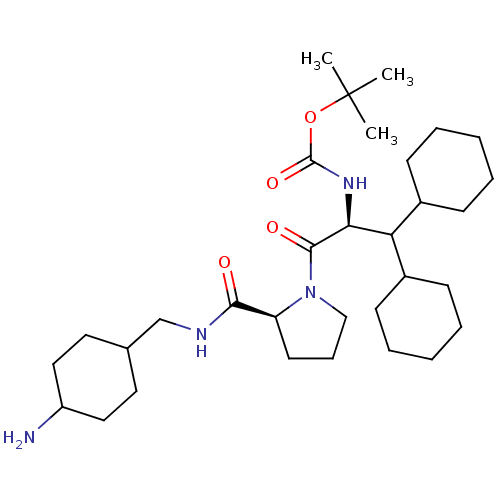
(((S)-2-{(S)-2-[(4-Amino-cyclohexylmethyl)-carbamoy...)Show SMILES CC(C)(C)OC(=O)N[C@@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:8.7,28.31,(4.89,-7.9,;5.69,-6.58,;4.94,-5.24,;6.75,-7.68,;7.68,-6.97,;8.02,-5.27,;7.26,-3.92,;9.56,-5.29,;10.89,-4.53,;10.89,-2.99,;9.56,-2.21,;9.56,-.67,;8.22,.1,;6.9,-.67,;6.9,-2.21,;8.22,-2.98,;12.23,-2.22,;13.55,-2.99,;14.9,-2.22,;14.9,-.68,;13.57,.09,;12.22,-.68,;12.23,-5.3,;12.23,-6.84,;13.71,-4.89,;14.26,-3.44,;15.79,-3.51,;16.21,-5,;14.92,-5.85,;14.85,-7.39,;13.49,-8.1,;16.15,-8.21,;17.52,-7.51,;18.81,-8.32,;17.75,-9.44,;18.84,-10.92,;18.78,-12.17,;19.86,-13.25,;19.86,-11.05,;18.78,-9.66,)| Show InChI InChI=1S/C32H56N4O4/c1-32(2,3)40-31(39)35-28(27(23-11-6-4-7-12-23)24-13-8-5-9-14-24)30(38)36-20-10-15-26(36)29(37)34-21-22-16-18-25(33)19-17-22/h22-28H,4-21,33H2,1-3H3,(H,34,37)(H,35,39)/t22?,25?,26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060709

((S)-1-(3-Phenyl-3-pyridin-2-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccccn2)CC1 |wU:9.8,(6.95,-10.61,;5.99,-9.43,;5.61,-8.16,;6.25,-6.56,;4.9,-5.8,;3.53,-5.18,;3.37,-3.64,;1.96,-3.02,;.71,-3.93,;1.8,-1.5,;2.95,-.48,;2.31,.93,;.81,.77,;.49,-.74,;-.92,-1.34,;-1.08,-2.88,;-2.16,-.45,;-2.16,1.09,;-.85,1.85,;.49,1.09,;1.83,1.85,;1.83,3.39,;.49,4.16,;-.85,3.39,;-3.51,1.85,;-3.51,3.41,;-4.82,4.18,;-6.16,3.41,;-6.13,1.85,;-4.82,1.11,;5.32,-6.97,;4.68,-8.66,)| Show InChI InChI=1S/C26H34N4O2/c27-21-13-11-19(12-14-21)18-29-26(32)24-10-6-16-30(24)25(31)17-22(20-7-2-1-3-8-20)23-9-4-5-15-28-23/h1-5,7-9,15,19,21-22,24H,6,10-14,16-18,27H2,(H,29,32)/t19?,21?,22?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human serine protease (plasma Kallikrein)(89000*) |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50056771

((2S)-N-[(4-aminocyclohexyl)methyl]-1-[(2R)-2-(meth...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC[C@H]1CCC(N)CC1 |wU:16.18,21.22,wD:2.1,(5.43,-11.22,;5.29,-9.68,;6.56,-8.79,;6.43,-7.27,;5.03,-6.59,;3.77,-7.5,;2.37,-6.83,;2.25,-5.29,;3.51,-4.42,;4.91,-5.07,;7.95,-9.44,;8.08,-10.98,;9.21,-8.58,;9.21,-7.04,;10.73,-6.57,;11.61,-7.83,;10.67,-9.07,;11.12,-10.54,;10.07,-11.66,;12.62,-10.89,;13.67,-9.77,;13.22,-8.3,;13.39,-6.83,;12.84,-5.45,;13.92,-4.35,;13.15,-3.02,;13.92,-6.01,;14.44,-7.48,)| Show InChI InChI=1S/C22H34N4O2/c1-24-19(14-16-6-3-2-4-7-16)22(28)26-13-5-8-20(26)21(27)25-15-17-9-11-18(23)12-10-17/h2-4,6-7,17-20,24H,5,8-15,23H2,1H3,(H,25,27)/t17-,18?,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of the compound against bovine trypsin was determined |
Bioorg Med Chem Lett 7: 67-72 (1997)
Article DOI: 10.1016/S0960-894X(96)00583-5
BindingDB Entry DOI: 10.7270/Q2639PQM |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50060710

((S)-1-(3,3-Diphenyl-butyryl)-pyrrolidine-2-carboxy...)Show SMILES CC(CC(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1)(c1ccccc1)c1ccccc1 |wU:9.10,(5.27,-.24,;5.29,-1.78,;5.3,-3.32,;6.54,-4.21,;6.38,-5.75,;7.96,-3.6,;8.28,-2.1,;9.8,-1.94,;10.43,-3.35,;9.28,-4.37,;9.44,-5.91,;8.19,-6.81,;10.84,-6.52,;11,-8.06,;12.39,-8.71,;12.8,-9.86,;12.17,-11.56,;13.49,-12.33,;14.45,-13.52,;13.09,-11.08,;13.73,-9.48,;6.62,-1.01,;6.62,.53,;7.96,1.3,;9.3,.53,;9.3,-1.01,;7.97,-1.78,;3.96,-1.01,;3.96,.53,;2.64,1.3,;1.29,.53,;1.32,-1.01,;2.64,-1.78,)| Show InChI InChI=1S/C28H37N3O2/c1-28(22-9-4-2-5-10-22,23-11-6-3-7-12-23)19-26(32)31-18-8-13-25(31)27(33)30-20-21-14-16-24(29)17-15-21/h2-7,9-12,21,24-25H,8,13-20,29H2,1H3,(H,30,33)/t21?,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50060711

((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease TPA |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human serine protease Coagulation factor X (1.7*10e6*) |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human serine protease (TPA) (2.5*10e6*) |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human serine protease (plasmin) (3.0*10e6) |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50060711

((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease plasma kallikrein |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50060711

((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease plasmin |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50060711

((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(C2CCCCC2)C2CCCCC2)CC1 |wU:9.8,(15.67,-13.97,;14.71,-12.78,;14.32,-11.52,;14.96,-9.91,;13.61,-9.14,;12.23,-8.51,;12.07,-6.97,;10.66,-6.35,;9.41,-7.26,;10.5,-4.82,;11.66,-3.8,;11.02,-2.39,;9.51,-2.55,;9.19,-4.05,;7.77,-4.66,;7.61,-6.2,;6.52,-3.76,;6.52,-2.22,;7.84,-1.44,;9.19,-2.21,;10.5,-1.44,;10.5,.1,;9.15,.87,;7.84,.09,;5.18,-1.45,;5.18,.1,;3.83,.87,;2.51,.1,;2.51,-1.45,;3.83,-2.22,;14.03,-10.31,;13.39,-12.01,)| Show InChI InChI=1S/C27H47N3O2/c28-23-15-13-20(14-16-23)19-29-27(32)25-12-7-17-30(25)26(31)18-24(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h20-25H,1-19,28H2,(H,29,32)/t20?,23?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease Coagulation factor X |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50060704

((S)-1-(2-5H-Dibenzo[a,d]cyclohepten-5-yl-acetyl)-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC2c3ccccc3C=Cc3ccccc23)CC1 |wU:9.8,c:26,(10.27,-10.54,;9.32,-9.36,;8.93,-8.11,;9.57,-6.52,;8.23,-5.75,;6.86,-5.12,;6.7,-3.58,;5.27,-2.98,;4.05,-3.87,;5.12,-1.45,;6.29,-.43,;5.65,.98,;4.15,.82,;3.83,-.68,;2.42,-1.29,;2.27,-2.82,;1.18,-.39,;1.18,1.14,;-.35,1.17,;-.93,-.27,;-2.46,-.49,;-3.41,.72,;-2.84,2.16,;-1.31,2.38,;-.96,3.88,;.45,4.52,;1.82,3.82,;2.94,4.87,;4.4,4.42,;4.75,2.92,;3.61,1.87,;2.14,2.32,;8.65,-6.9,;8.01,-8.59,)| Show InChI InChI=1S/C29H35N3O2/c30-23-15-11-20(12-16-23)19-31-29(34)27-10-5-17-32(27)28(33)18-26-24-8-3-1-6-21(24)13-14-22-7-2-4-9-25(22)26/h1-4,6-9,13-14,20,23,26-27H,5,10-12,15-19,30H2,(H,31,34)/t20?,23?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease TPA |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50060709

((S)-1-(3-Phenyl-3-pyridin-2-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccccn2)CC1 |wU:9.8,(6.95,-10.61,;5.99,-9.43,;5.61,-8.16,;6.25,-6.56,;4.9,-5.8,;3.53,-5.18,;3.37,-3.64,;1.96,-3.02,;.71,-3.93,;1.8,-1.5,;2.95,-.48,;2.31,.93,;.81,.77,;.49,-.74,;-.92,-1.34,;-1.08,-2.88,;-2.16,-.45,;-2.16,1.09,;-.85,1.85,;.49,1.09,;1.83,1.85,;1.83,3.39,;.49,4.16,;-.85,3.39,;-3.51,1.85,;-3.51,3.41,;-4.82,4.18,;-6.16,3.41,;-6.13,1.85,;-4.82,1.11,;5.32,-6.97,;4.68,-8.66,)| Show InChI InChI=1S/C26H34N4O2/c27-21-13-11-19(12-14-21)18-29-26(32)24-10-6-16-30(24)25(31)17-22(20-7-2-1-3-8-20)23-9-4-5-15-28-23/h1-5,7-9,15,19,21-22,24H,6,10-14,16-18,27H2,(H,29,32)/t19?,21?,22?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease Coagulation factor X |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50060703

((S)-1-(3-Phenyl-3-pyridin-4-yl-propionyl)-pyrrolid...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2ccncc2)CC1 |wU:9.8,(4.43,-12.54,;3.47,-11.35,;3.06,-10.09,;3.71,-8.48,;2.38,-7.71,;.97,-7.08,;.81,-5.54,;-.58,-4.92,;-1.83,-5.83,;-.74,-3.38,;.4,-2.36,;-.22,-.94,;-1.76,-1.1,;-2.08,-2.61,;-3.49,-3.22,;-3.65,-4.77,;-4.75,-2.32,;-4.75,-.78,;-3.41,-.01,;-2.06,-.78,;-.73,-.01,;-.73,1.53,;-2.08,2.3,;-3.41,1.53,;-6.07,-.01,;-6.07,1.54,;-7.39,2.31,;-8.74,1.54,;-8.73,-.01,;-7.39,-.77,;2.77,-8.88,;2.15,-10.58,)| Show InChI InChI=1S/C26H34N4O2/c27-22-10-8-19(9-11-22)18-29-26(32)24-7-4-16-30(24)25(31)17-23(20-5-2-1-3-6-20)21-12-14-28-15-13-21/h1-3,5-6,12-15,19,22-24H,4,7-11,16-18,27H2,(H,29,32)/t19?,22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease Coagulation factor X |
J Med Chem 40: 3687-93 (1997)
Article DOI: 10.1021/jm970397q
BindingDB Entry DOI: 10.7270/Q20Z73ZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































