

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50454822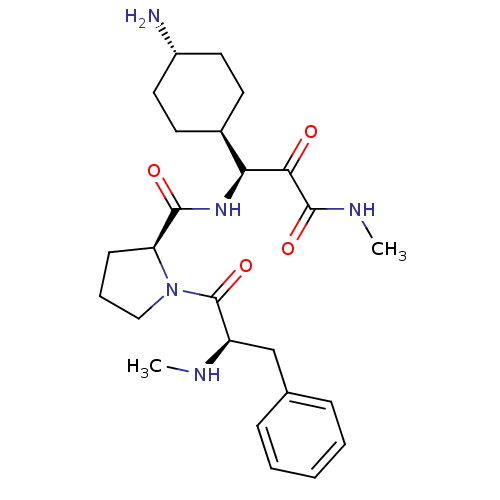 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057828 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063555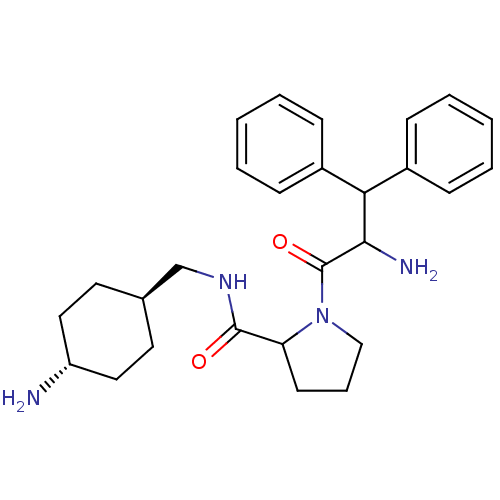 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066331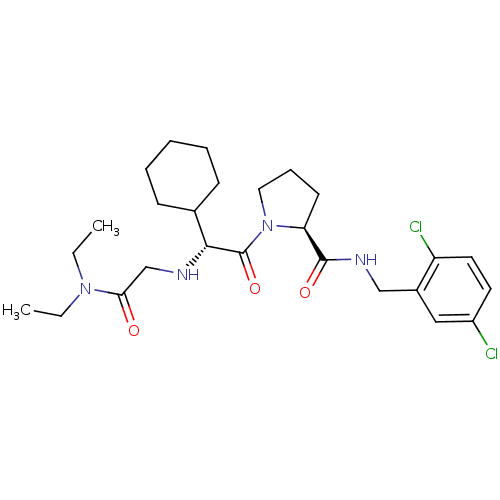 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066338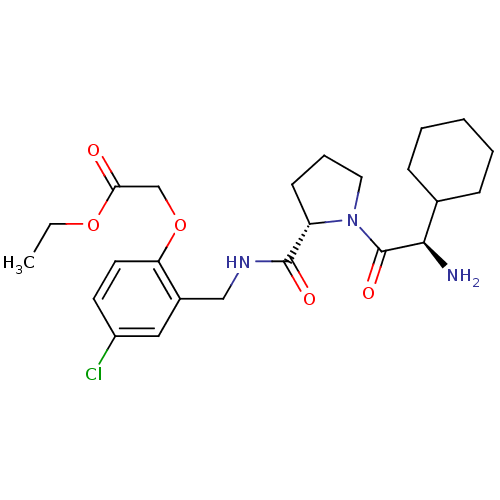 (CHEMBL327115 | [2-({[(S)-1-((R)-2-Amino-2-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066332 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066333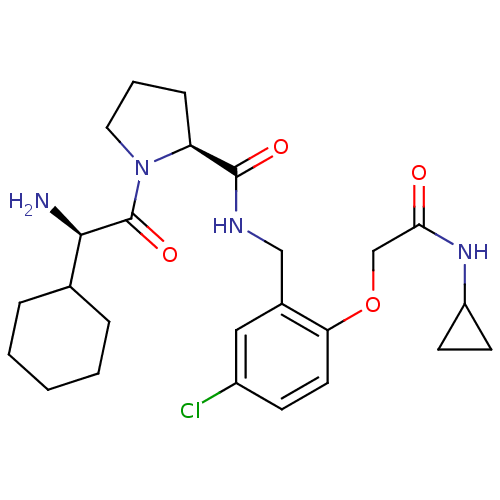 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066334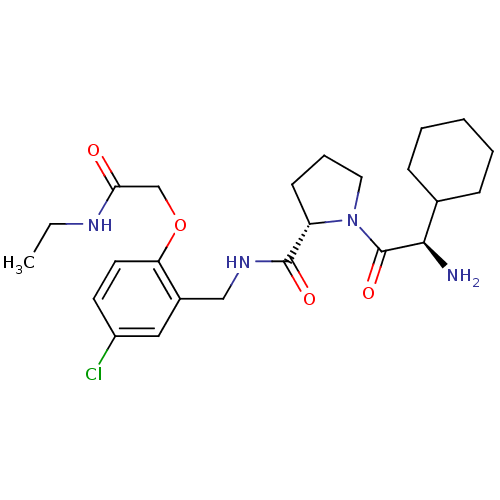 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164268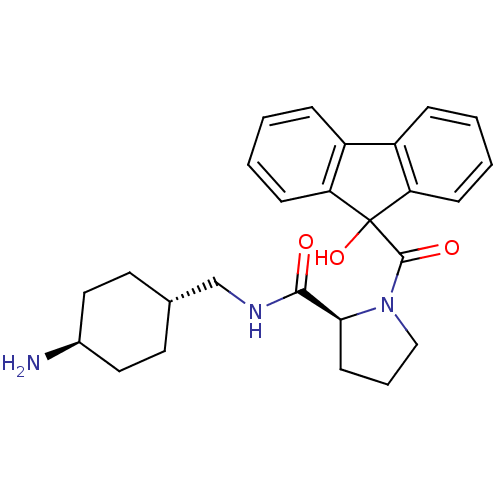 ((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062623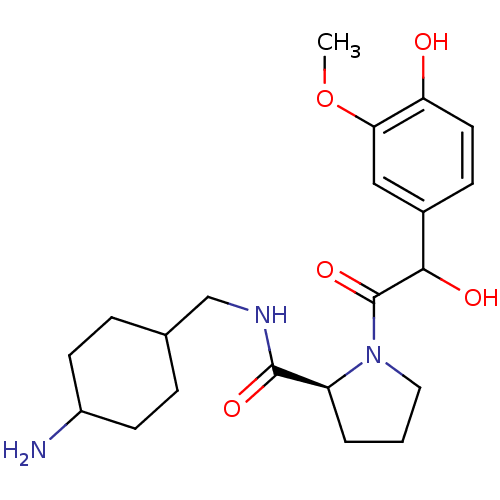 ((S)-1-[2-Hydroxy-2-(4-hydroxy-3-methoxy-phenyl)-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062634 ((S)-1-[3-(4-Hydroxy-phenyl)-2-phenyl-propionyl]-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066340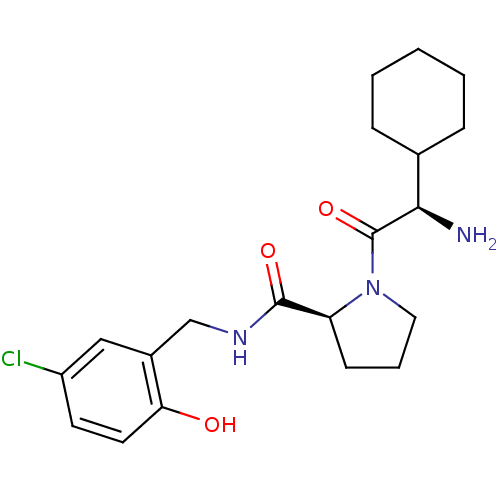 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066337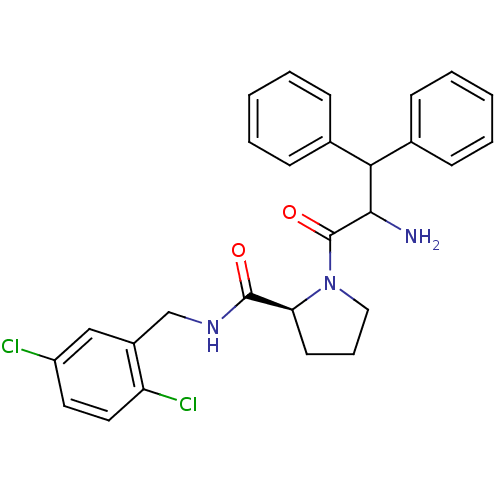 ((S)-1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063562 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062628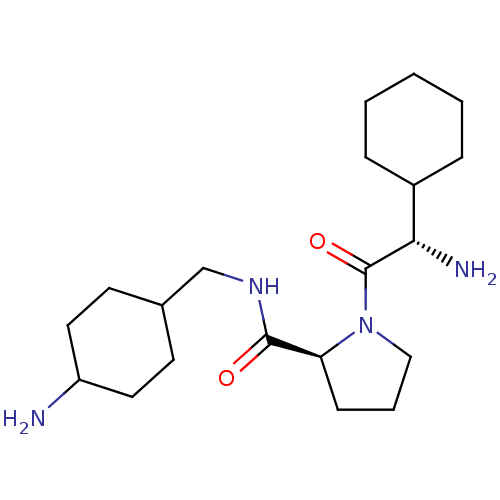 ((S)-1-((S)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366827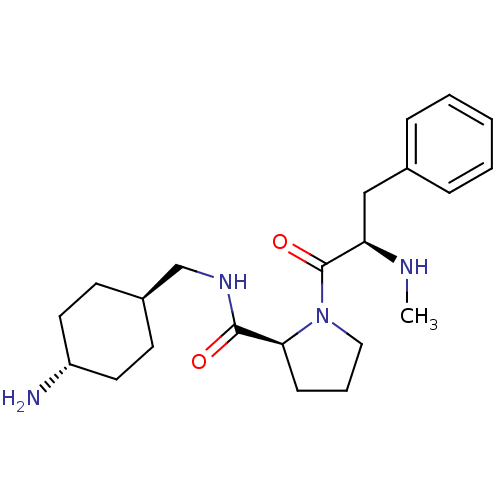 (CHEMBL125181 | L-371912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066336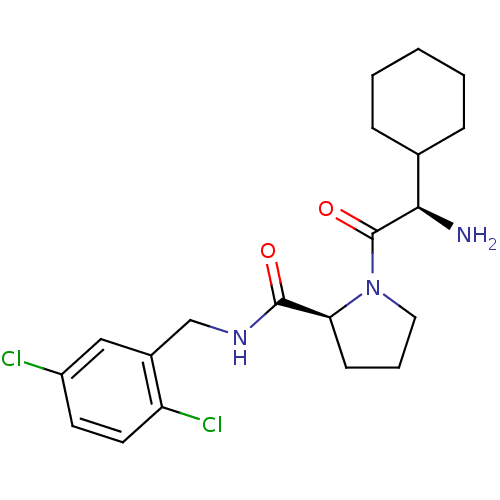 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066339 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066341 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063549 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066335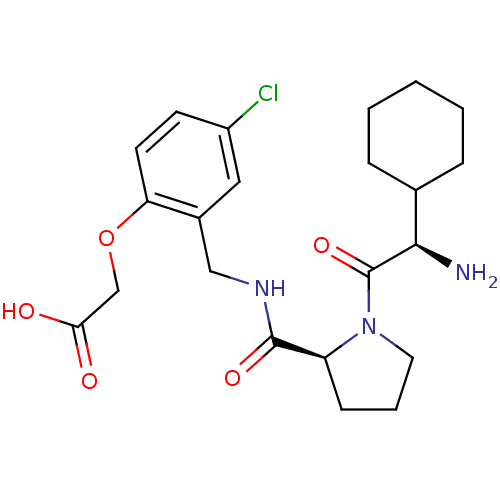 (CHEMBL111051 | [2-({[(S)-1-((R)-2-Amino-2-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063558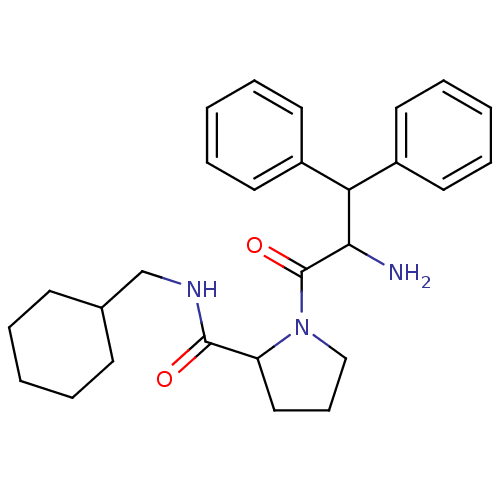 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062624 ((S)-1-(2,3-Dihydro-benzo[1,4]dioxine-2-carbonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063559 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50164268 ((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards trypsin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50139111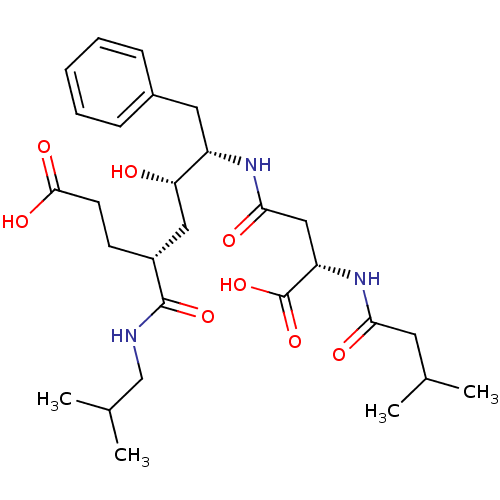 ((4R,6S,7S)-7-[(S)-3-Carboxy-3-(3-methyl-butyrylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of Beta-secretase 1 (BACE 1) expressed in insect cell using baculovirus | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50139112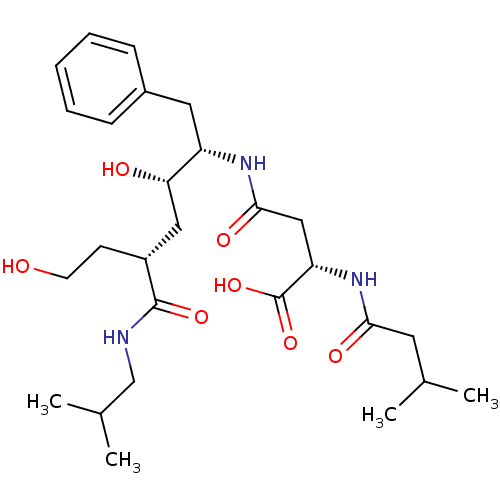 ((S)-N-((1S,2S,4R)-1-Benzyl-2,6-dihydroxy-4-isobuty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of Beta-secretase 1 (BACE 1) expressed in insect cell using baculovirus | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50139109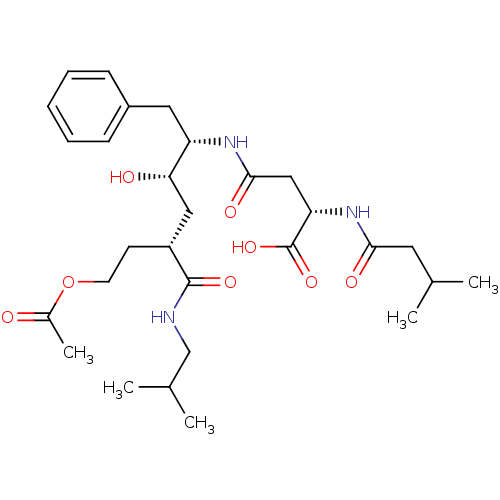 ((S)-N-((1S,2S,4R)-6-Acetoxy-1-benzyl-2-hydroxy-4-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of human renin | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50139109 ((S)-N-((1S,2S,4R)-6-Acetoxy-1-benzyl-2-hydroxy-4-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of cathepsin D | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50139109 ((S)-N-((1S,2S,4R)-6-Acetoxy-1-benzyl-2-hydroxy-4-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of Beta-secretase 1 (BACE 1) expressed in insect cell using baculovirus | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066337 ((S)-1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Selectivity of the compound was evaluated as the binding affinity between thrombin versus trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066332 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50139112 ((S)-N-((1S,2S,4R)-1-Benzyl-2,6-dihydroxy-4-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of human renin | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50066332 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease TPA. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50139112 ((S)-N-((1S,2S,4R)-1-Benzyl-2,6-dihydroxy-4-isobuty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of cathepsin D | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066333 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50066336 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease TPA. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066334 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50139110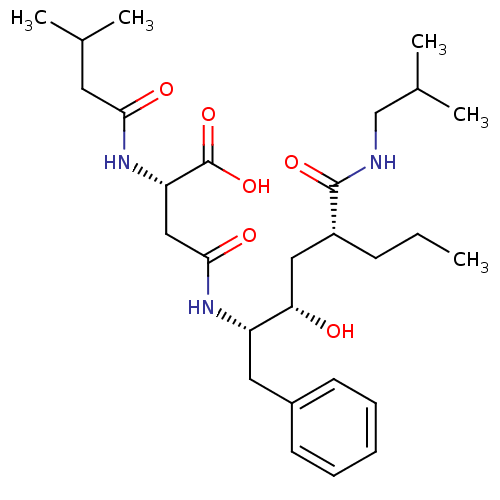 ((S)-N-((1S,2S,4R)-1-Benzyl-2-hydroxy-4-isobutylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of human renin | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066331 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50139110 ((S)-N-((1S,2S,4R)-1-Benzyl-2-hydroxy-4-isobutylcar...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of cathepsin D | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50066336 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease trypsin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50066331 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease TPA. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50066333 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease TPA. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066336 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease Chymotrypsinogen | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066337 ((S)-1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Selectivity of the compound was evaluated as the binding affinity between thrombin versus Chymotrypsinogen | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066333 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease Chymotrypsinogen | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50139110 ((S)-N-((1S,2S,4R)-1-Benzyl-2-hydroxy-4-isobutylcar...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of Beta-secretase 1 (BACE 1) expressed in insect cell using baculovirus | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066334 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease Chymotrypsinogen | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50139111 ((4R,6S,7S)-7-[(S)-3-Carboxy-3-(3-methyl-butyrylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of human renin | Bioorg Med Chem Lett 14: 601-4 (2004) BindingDB Entry DOI: 10.7270/Q21835X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |