

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822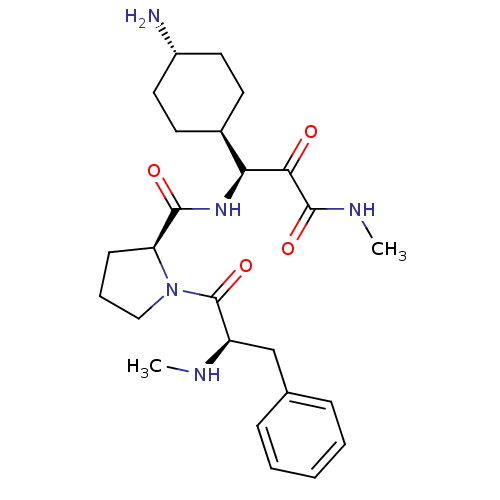 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057828 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069189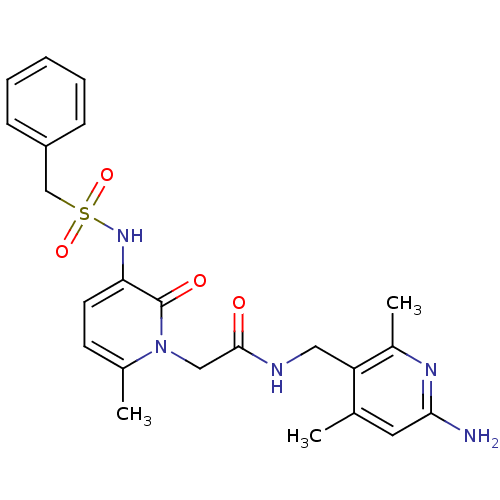 (CHEMBL353431 | N-(6-Amino-2,4-dimethyl-pyridin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067795 (CHEMBL138855 | N-(6-Amino-2,4-dimethyl-pyridin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796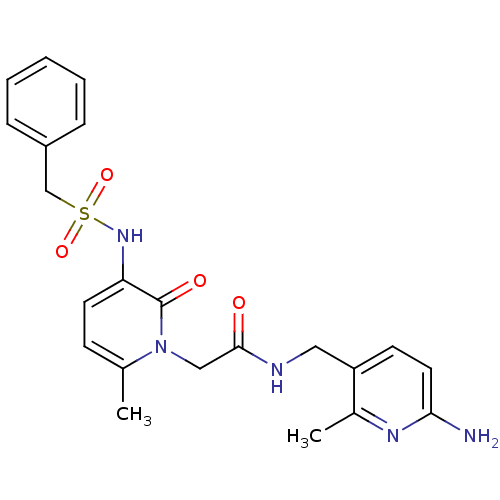 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069190 (CHEMBL287614 | N-(1-Carbamimidoyl-piperidin-4-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067798 (CHEMBL336438 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797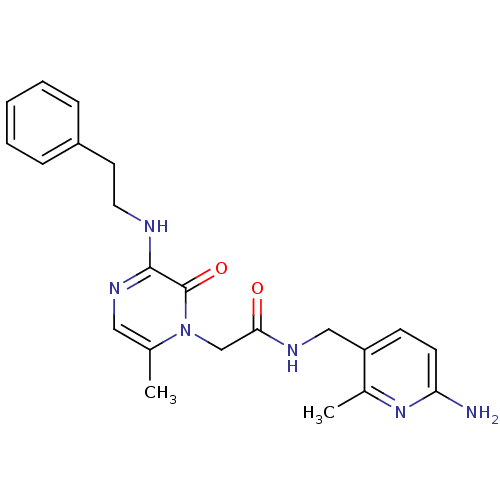 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164268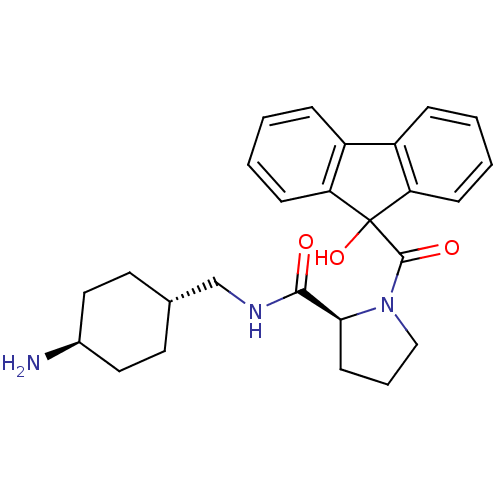 ((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069191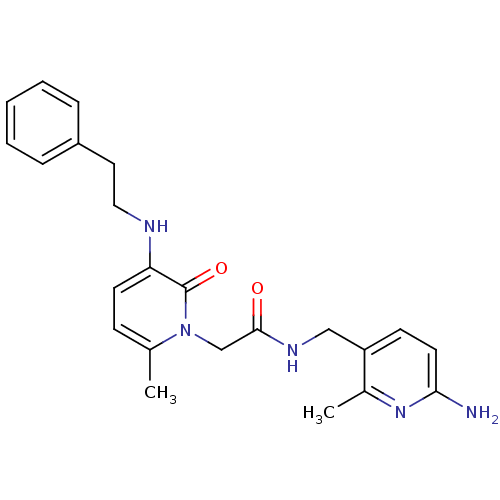 (CHEMBL349430 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062623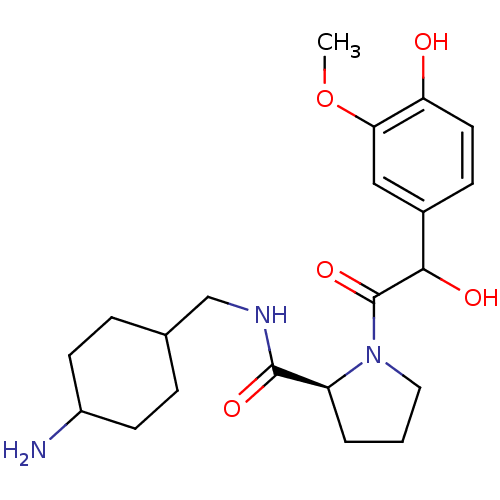 ((S)-1-[2-Hydroxy-2-(4-hydroxy-3-methoxy-phenyl)-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062634 ((S)-1-[3-(4-Hydroxy-phenyl)-2-phenyl-propionyl]-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062628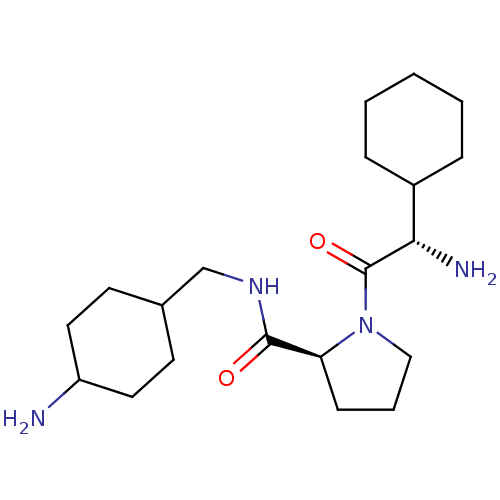 ((S)-1-((S)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366827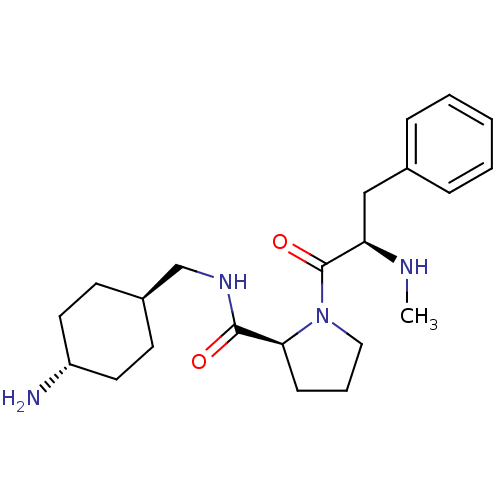 (CHEMBL125181 | L-371912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067791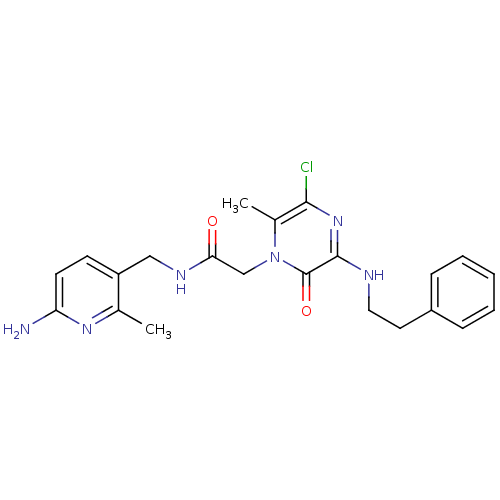 (CHEMBL140885 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067793 (CHEMBL139331 | N-(6-Amino-pyridin-3-ylmethyl)-2-(6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069194 (CHEMBL355441 | N-(6-Amino-pyridin-3-ylmethyl)-2-(6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060751 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069195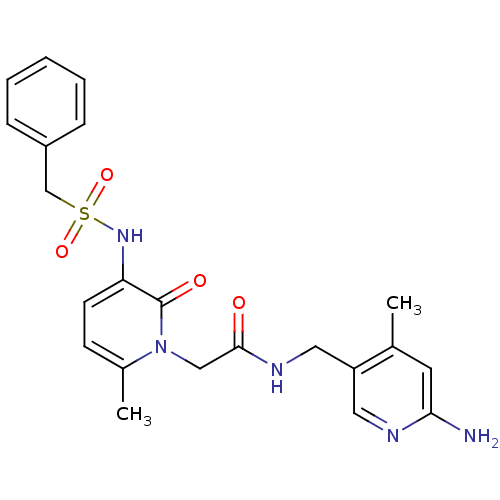 (CHEMBL354555 | N-(6-Amino-4-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM26621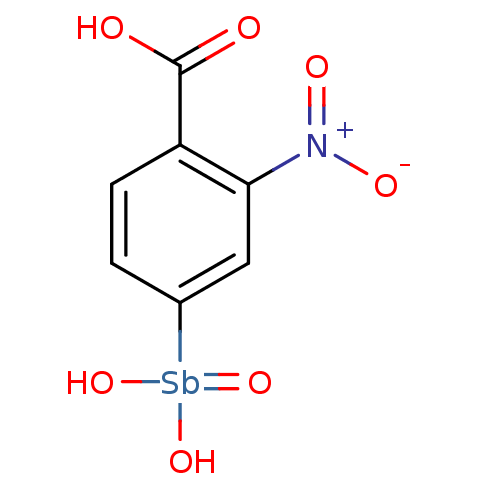 (4-[dihydroxy(oxo)--stibanyl]-2-nitrobenzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 19 | -43.6 | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Johns Hopkins University | Assay Description The screening assay was performed using 384-well microtiter plate first spotted with test compounds. Then Ape1 in reaction buffer was added to each w... | Mol Pharmacol 73: 669-77 (2008) Article DOI: 10.1124/mol.107.042622 BindingDB Entry DOI: 10.7270/Q2WS8RJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067794 (CHEMBL139178 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067792 (CHEMBL141266 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069192 (CHEMBL48929 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM26622 (Compound 13793 | ethyl 4-{4-[dihydroxy(oxo)--stiba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 36 | -42.1 | 17 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Johns Hopkins University | Assay Description The screening assay was performed using 384-well microtiter plate first spotted with test compounds. Then Ape1 in reaction buffer was added to each w... | Mol Pharmacol 73: 669-77 (2008) Article DOI: 10.1124/mol.107.042622 BindingDB Entry DOI: 10.7270/Q2WS8RJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069193 (CHEMBL424545 | N-(4-Amino-cyclohexylmethyl)-2-(6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was evaluated | Bioorg Med Chem Lett 8: 817-22 (1999) BindingDB Entry DOI: 10.7270/Q2JH3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113742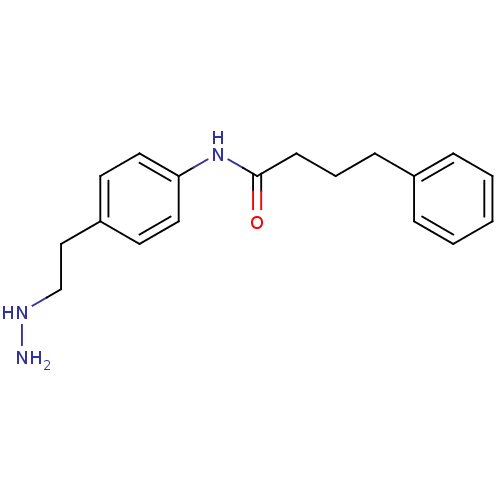 (N-[4-(2-Hydrazinylethyl)phenyl]-4-phenylbutanamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50062624 ((S)-1-(2,3-Dihydro-benzo[1,4]dioxine-2-carbonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113753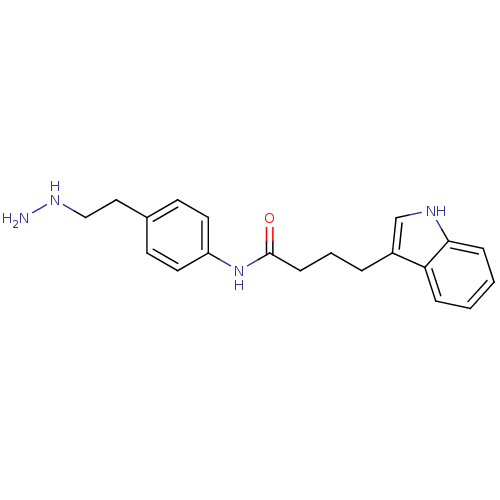 (N-[4-(2-Hydrazinylethyl)phenyl]-4-(1H-indol-3-yl)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113750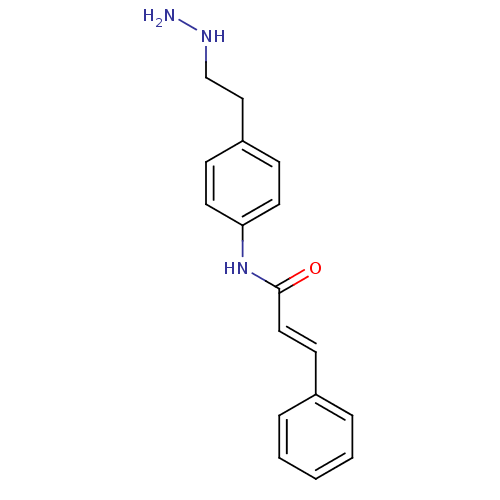 ((2E)-N-[4-(2-Hydrazinylethyl)phenyl]-3-phenylprop-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113745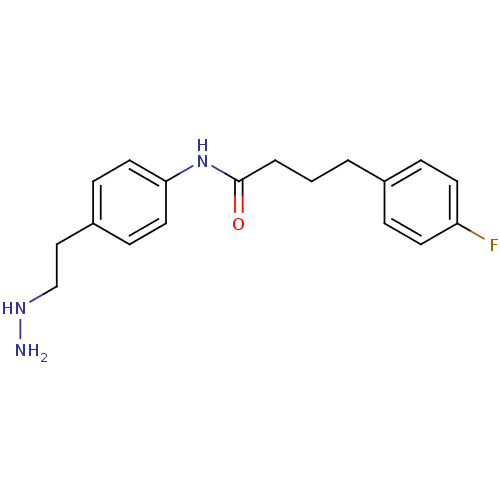 (4-(4-Fluorophenyl)-N-[4-(2-hydrazinylethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113744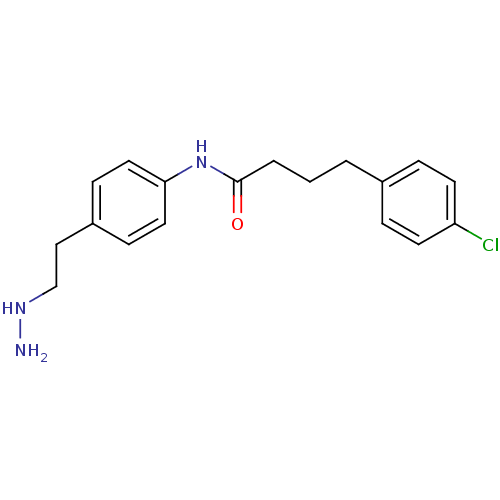 (4-(4-Chlorophenyl)-N-[4-(2-hydrazinylethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha/beta/gamma/delta/epsilon/eta/theta type/Serine/threonine-protein kinase D1/D3 (Homo sapiens (Human)) | BDBM50368315 (PROSTRATIN) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute (NCI) Curated by ChEMBL | Assay Description Displacement of [3H]PDBu binding to Protein kinase C of CEM cells with 10% fetal calf serum | J Med Chem 35: 1978-86 (1992) BindingDB Entry DOI: 10.7270/Q22Z17SG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113748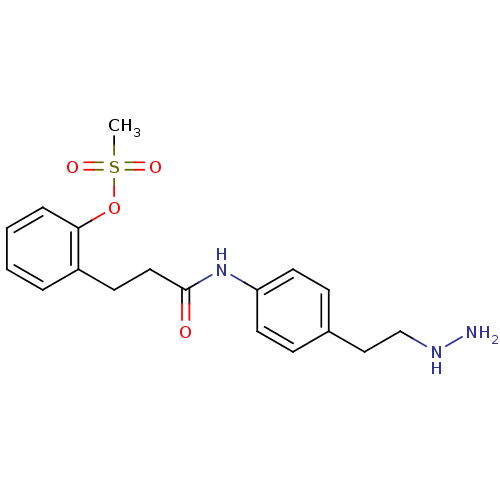 (2-(3-{[4-(2-Hydrazinylethyl)phenyl]amino}-3-oxopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1107 total ) | Next | Last >> |