| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50164654 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_302539 (CHEMBL875215) |
|---|
| Ki | 37±n/a nM |
|---|
| Citation |  Boyle, NA; Rajwanshi, VK; Prhavc, M; Wang, G; Fagan, P; Chen, F; Ewing, GJ; Brooks, JL; Hurd, T; Leeds, JM; Bruice, TW; Cook, PD Synthesis of 2',3'-dideoxynucleoside 5'-alpha-P-borano-beta,gamma-(difluoromethylene)triphosphates and their inhibition of HIV-1 reverse transcriptase. J Med Chem48:2695-700 (2005) [PubMed] Article Boyle, NA; Rajwanshi, VK; Prhavc, M; Wang, G; Fagan, P; Chen, F; Ewing, GJ; Brooks, JL; Hurd, T; Leeds, JM; Bruice, TW; Cook, PD Synthesis of 2',3'-dideoxynucleoside 5'-alpha-P-borano-beta,gamma-(difluoromethylene)triphosphates and their inhibition of HIV-1 reverse transcriptase. J Med Chem48:2695-700 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50164654 |
|---|
| n/a |
|---|
| Name | BDBM50164654 |
|---|
| Synonyms: | CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropurin-9-yl)-1-cyclopent-2-enyl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxyphosphonic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H16N5O11P3 |
|---|
| Mol. Mass. | 487.1929 |
|---|
| SMILES | Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C=C1 |c:30| |
|---|
| Structure | 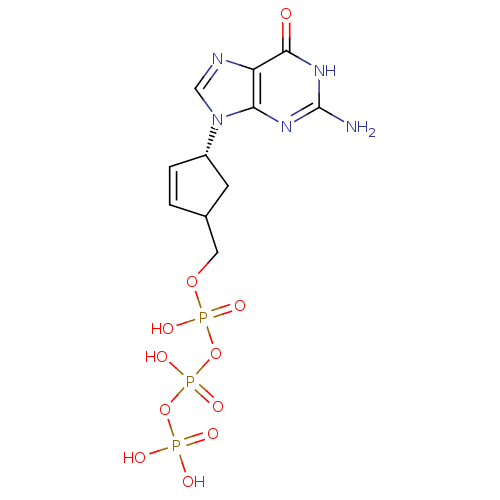
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Boyle, NA; Rajwanshi, VK; Prhavc, M; Wang, G; Fagan, P; Chen, F; Ewing, GJ; Brooks, JL; Hurd, T; Leeds, JM; Bruice, TW; Cook, PD Synthesis of 2',3'-dideoxynucleoside 5'-alpha-P-borano-beta,gamma-(difluoromethylene)triphosphates and their inhibition of HIV-1 reverse transcriptase. J Med Chem48:2695-700 (2005) [PubMed] Article
Boyle, NA; Rajwanshi, VK; Prhavc, M; Wang, G; Fagan, P; Chen, F; Ewing, GJ; Brooks, JL; Hurd, T; Leeds, JM; Bruice, TW; Cook, PD Synthesis of 2',3'-dideoxynucleoside 5'-alpha-P-borano-beta,gamma-(difluoromethylene)triphosphates and their inhibition of HIV-1 reverse transcriptase. J Med Chem48:2695-700 (2005) [PubMed] Article