

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164642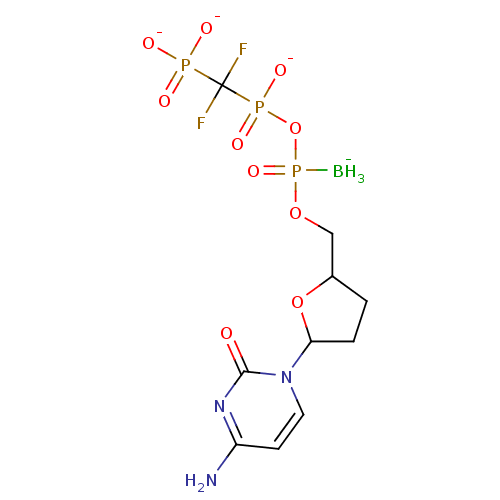 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164639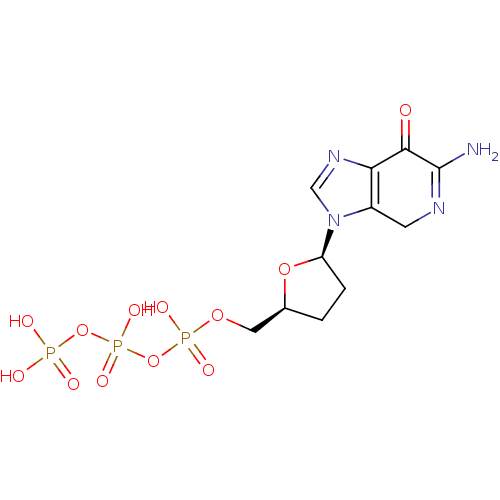 (2'-3'-dideoxy-7-deaza-guaninetriphosphate | CHEMBL...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164652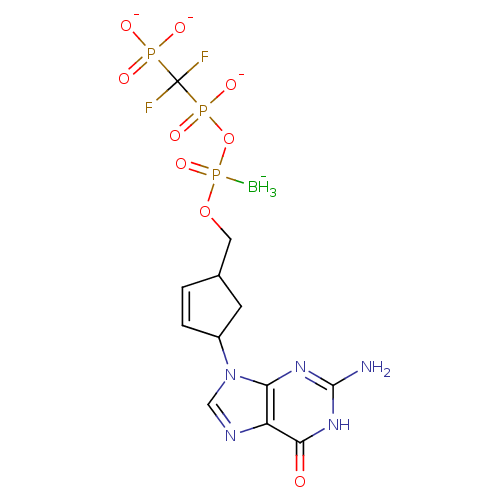 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164654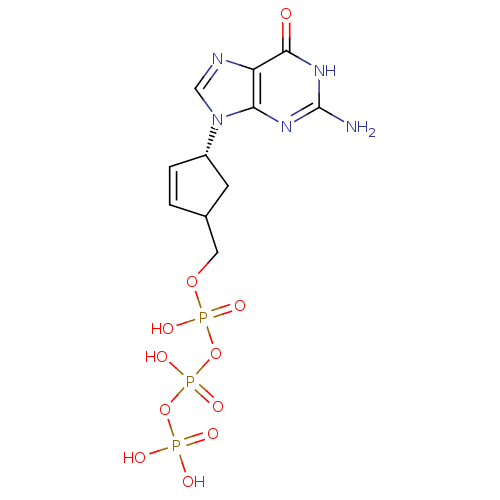 (CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropuri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164637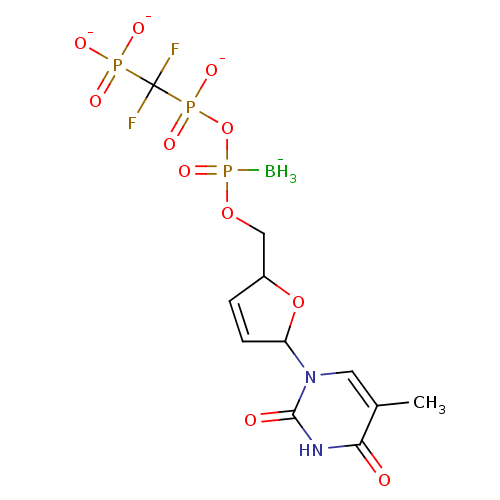 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164638 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164647 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50145605 (4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370655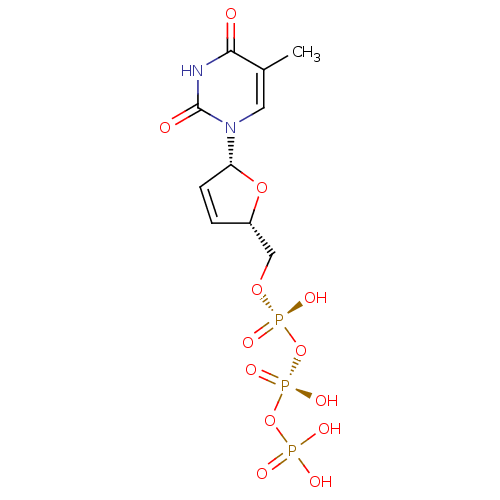 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164648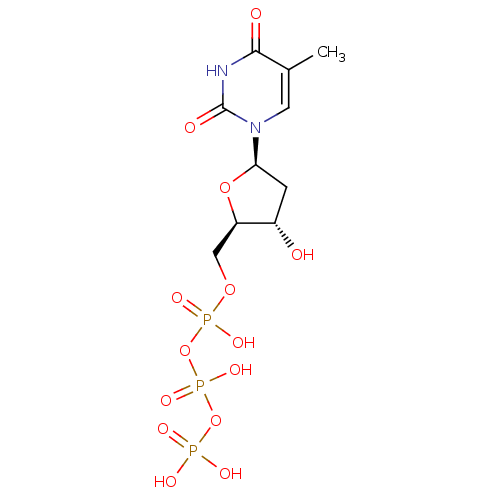 (2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164653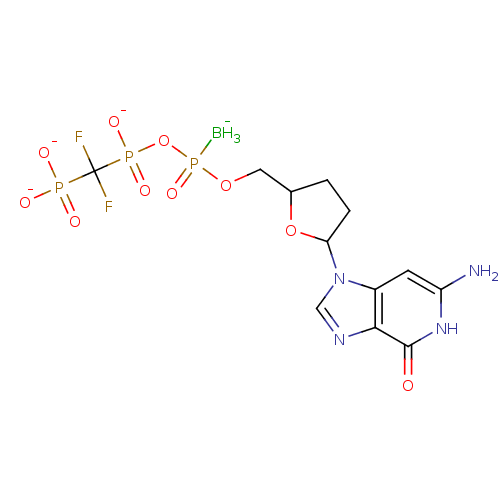 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164650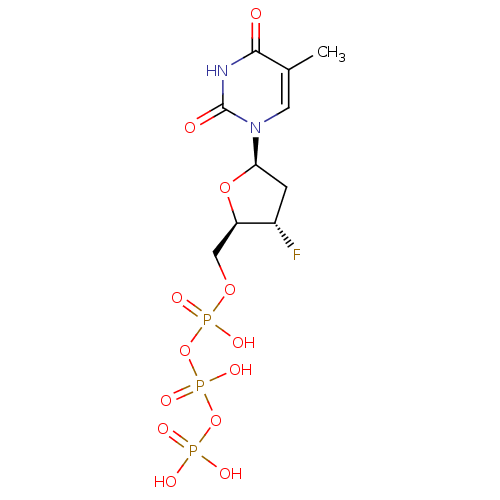 (CHEMBL191725 | [[[3-fluoro-5-(5-methyl-2,4-dioxo-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164640 (CHEMBL190609 | [[[[3-azido-5-(5-methyl-2,4-dioxo-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50138406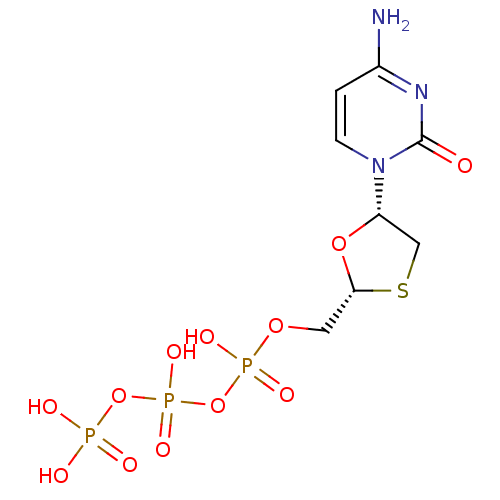 (3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamiv...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164655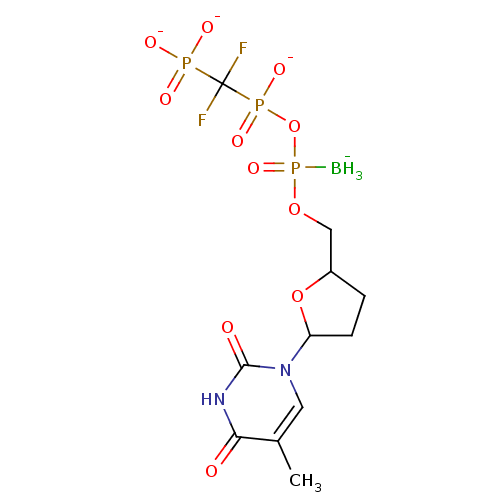 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164651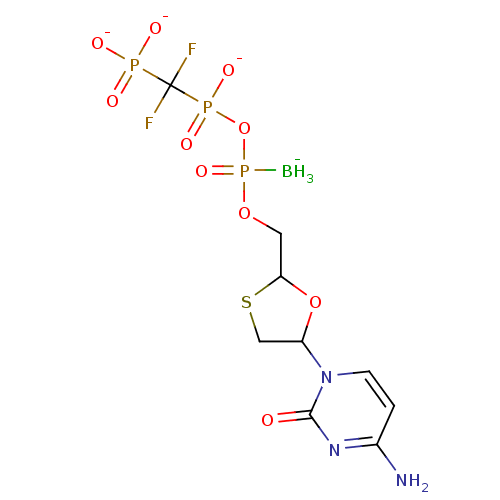 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164656 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164645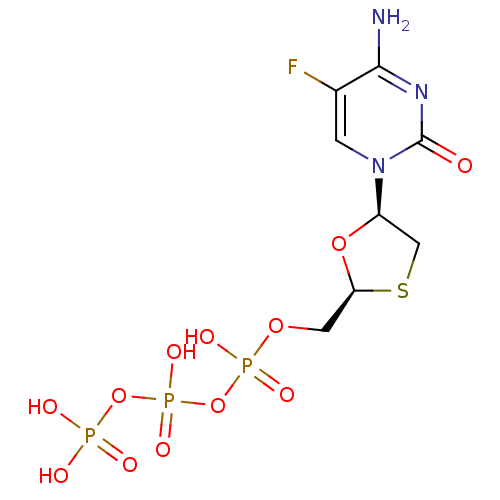 (CHEMBL192771 | [[[5-(4-amino-5-fluoro-2-oxo-1H-pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164646 (CHEMBL192371 | [[[5-(2,4-dioxo-1H-pyrimidin-1-yl)t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164643 (({[({[5-(4-amino-5-fluoro-2-oxo-1,2-dihydropyrimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544633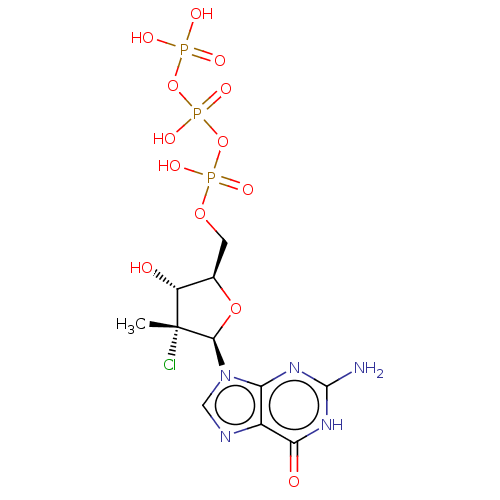 (CHEMBL4641646) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50332459 (((2R,3R,4R,5R)-5-(2-amino-6-oxo-1H-purin-9(6H)-yl)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544634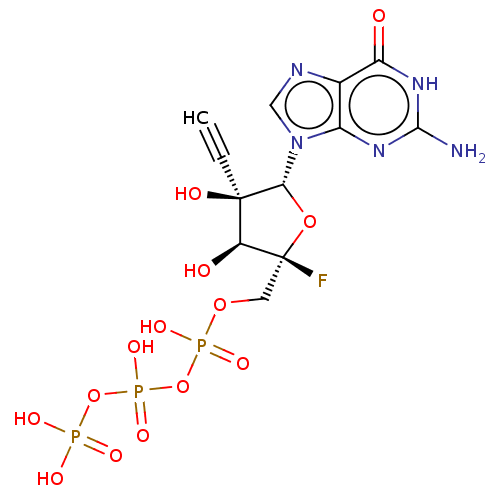 (CHEMBL4632955) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM466313 (US10793591, Compound 9) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM466319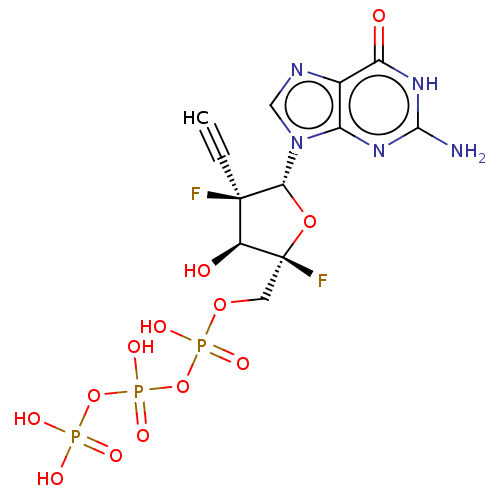 (US10793591, Compound 44) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544635 (CHEMBL4640290) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544637 (CHEMBL4635006) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544632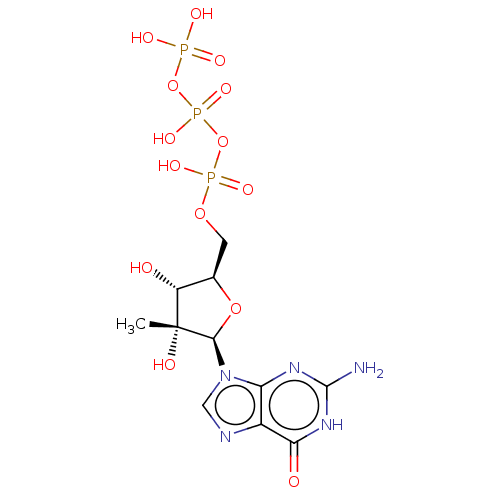 (CHEMBL3422648) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544636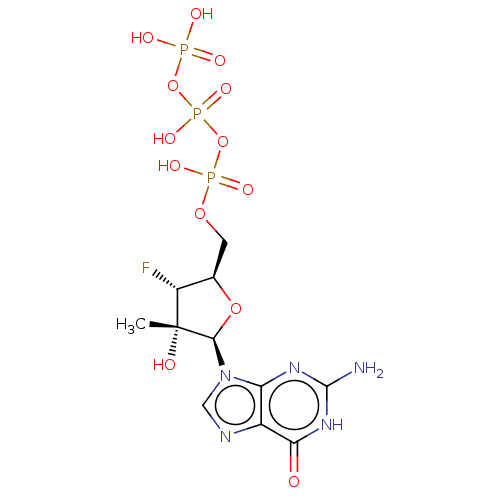 (CHEMBL4632867) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50544638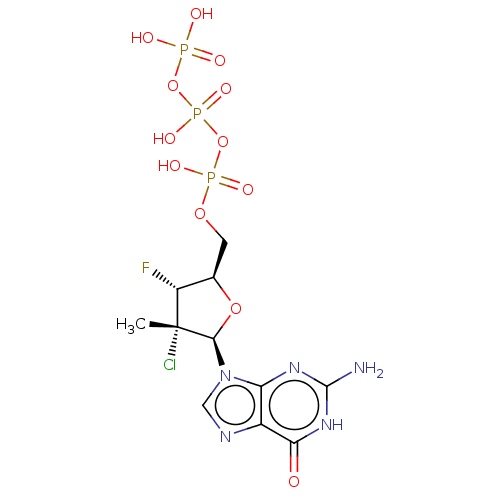 (CHEMBL4639840) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen BioPharma, Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA-dependent RNA polymerase activity assessed as reduction in radiolabeled ribonucleotide monophosphates incorporation into R... | J Med Chem 63: 10380-10395 (2020) Article DOI: 10.1021/acs.jmedchem.0c00935 BindingDB Entry DOI: 10.7270/Q2TM7FP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217798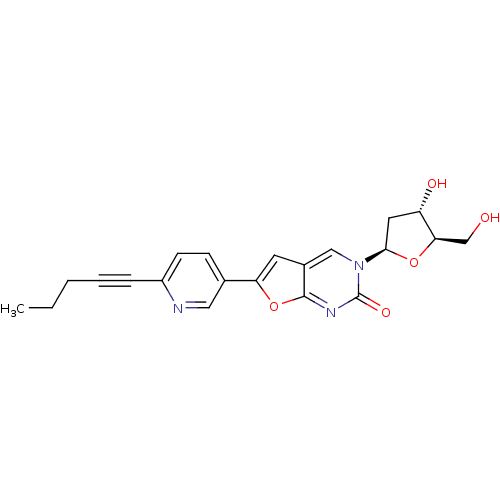 (3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-[2-(pe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217802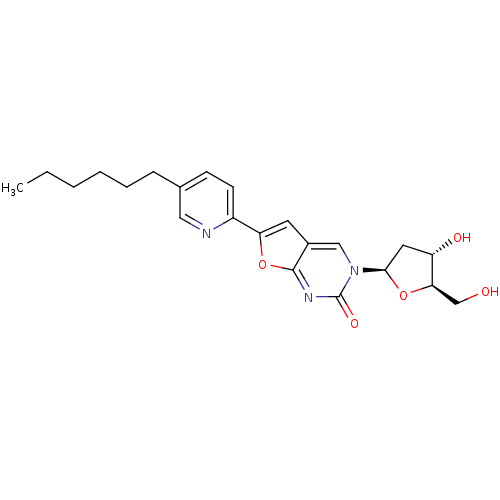 (3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-hex...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217799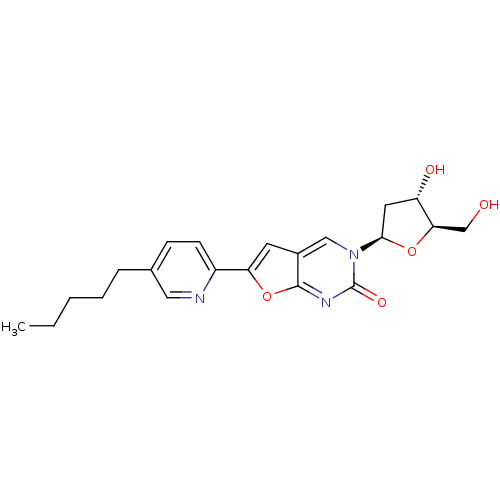 (3-(2-deoxy-D-erythro-pentofuranosyl)-6-(5-pentylpy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217806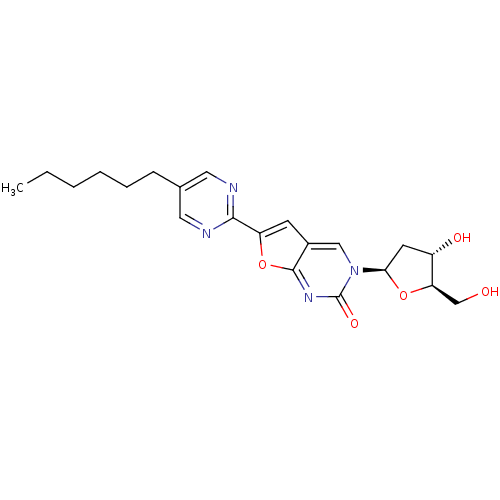 (3-(2-deoxy-D-erythro-pentofuranosyl)-6-(2-hexylpyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217797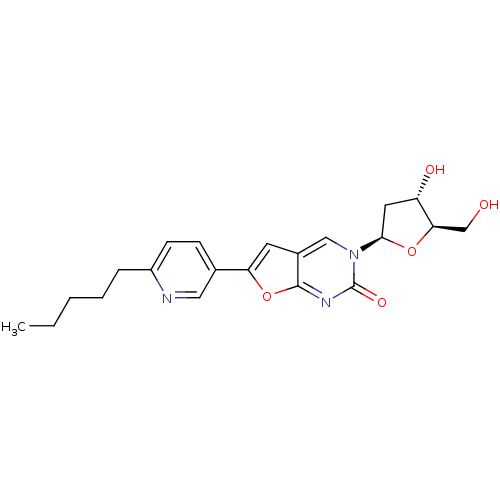 (3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(2-pen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217810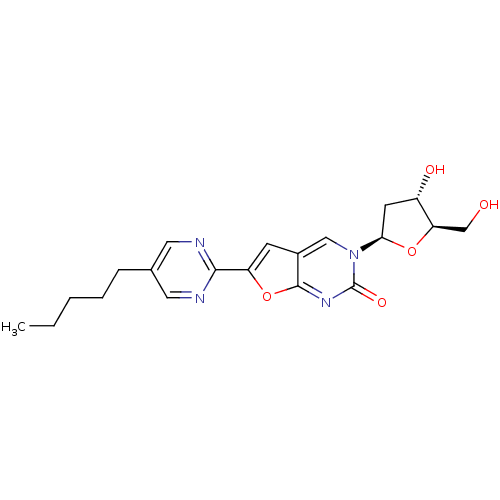 (3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(5-pen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217804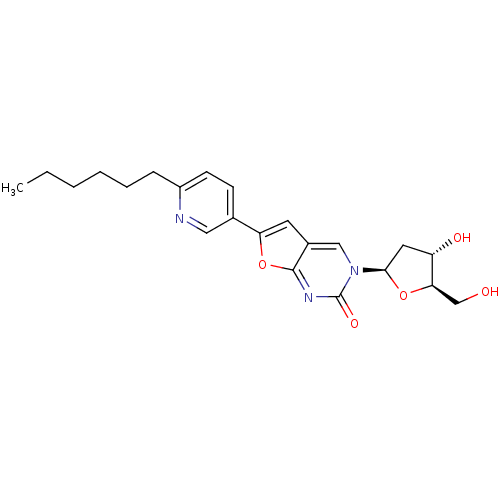 (3-(2-deoxy-D-erythro-pentofuranosyl)-6-(2-hexylpyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217809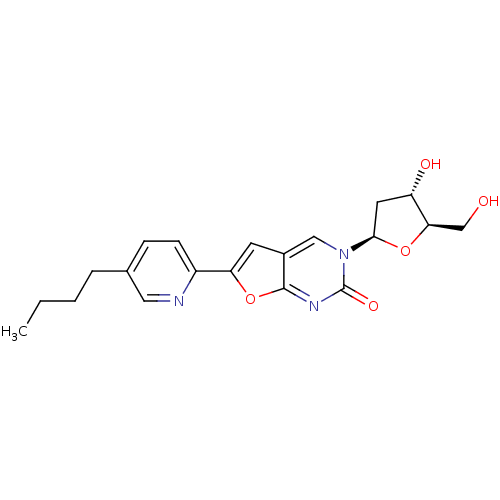 (6-(5-butylpyrid-2-yl)-3-(2-deoxy-beta-D-erythro-pe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217807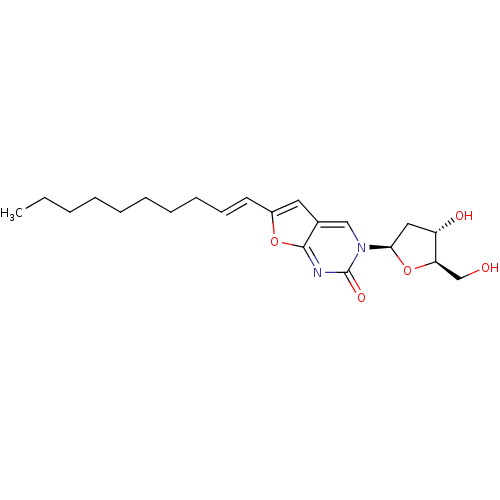 ((E)-6-(dec-1-en-1-yl)-3-(2-deoxy-beta-D-erythro-pe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50180316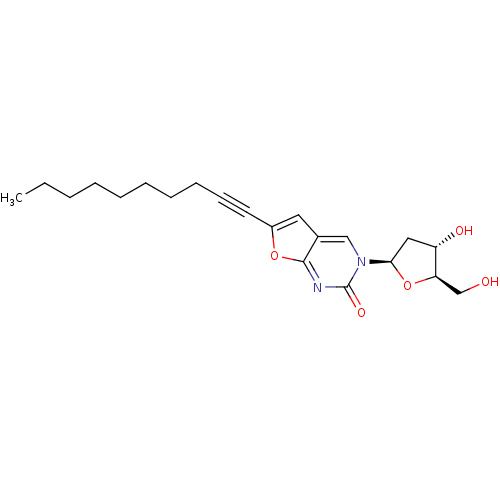 (3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-decyn-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217795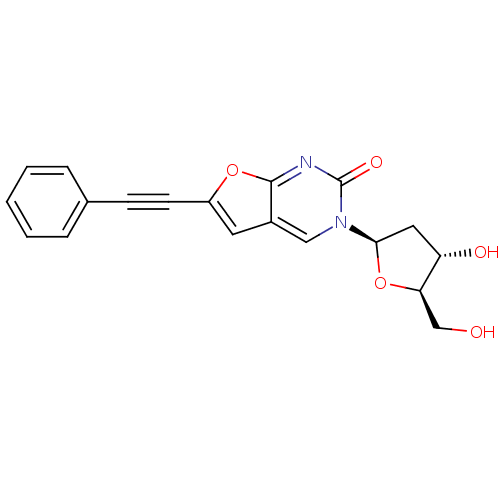 (3-(2-deoxy-beta-d-erythro-pentofuranosyl)-6-(pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217796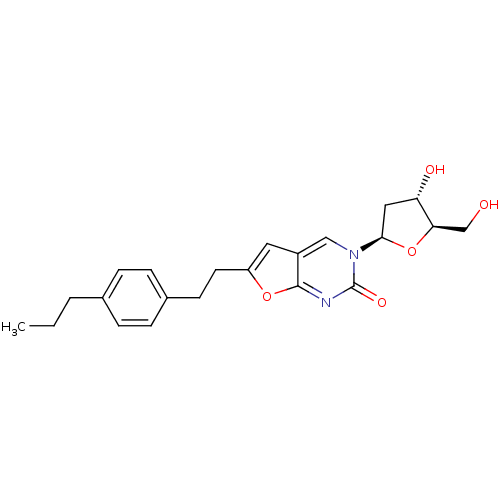 (3-(2-deoxy-D-erythro-pentofuranosyl)-6-[2-(4-propy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217803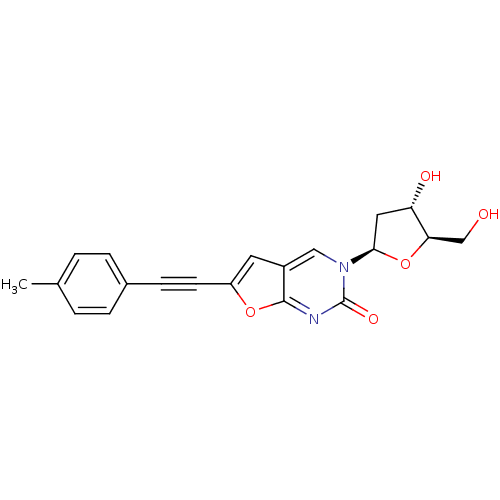 (3-(2-deoxy-D-erythro-pentofuranosyl)-6-[(4-methylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of VZV thymidine kinase | J Med Chem 50: 3897-905 (2007) Article DOI: 10.1021/jm070210n BindingDB Entry DOI: 10.7270/Q2S75G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||