| Reaction Details |
|---|
 | Report a problem with these data |
| Target | NF-kappa-B inhibitor alpha |
|---|
| Ligand | BDBM50227494 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_465169 (CHEMBL946815) |
|---|
| EC50 | 17000±n/a nM |
|---|
| Citation |  Xie, Y; Deng, S; Thomas, CJ; Liu, Y; Zhang, YQ; Rinderspacher, A; Huang, W; Gong, G; Wyler, M; Cayanis, E; Aulner, N; Többen, U; Chung, C; Pampou, S; Southall, N; Vidovic, D; Schürer, S; Branden, L; Davis, RE; Staudt, LM; Inglese, J; Austin, CP; Landry, DW; Smith, DH; Auld, DS Identification of N-(quinolin-8-yl)benzenesulfonamides as agents capable of down-regulating NFkappaB activity within two separate high-throughput screens of NFkappaB activation. Bioorg Med Chem Lett18:329-35 (2008) [PubMed] Article Xie, Y; Deng, S; Thomas, CJ; Liu, Y; Zhang, YQ; Rinderspacher, A; Huang, W; Gong, G; Wyler, M; Cayanis, E; Aulner, N; Többen, U; Chung, C; Pampou, S; Southall, N; Vidovic, D; Schürer, S; Branden, L; Davis, RE; Staudt, LM; Inglese, J; Austin, CP; Landry, DW; Smith, DH; Auld, DS Identification of N-(quinolin-8-yl)benzenesulfonamides as agents capable of down-regulating NFkappaB activity within two separate high-throughput screens of NFkappaB activation. Bioorg Med Chem Lett18:329-35 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| NF-kappa-B inhibitor alpha |
|---|
| Name: | NF-kappa-B inhibitor alpha |
|---|
| Synonyms: | I-kappa-B-alpha | IKBA | IKBA_HUMAN | MAD3 | NF-kappa-B inhibitor alpha | NFKBI | NFKBIA |
|---|
| Type: | GST fusion protein |
|---|
| Mol. Mass.: | 35584.84 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | It was produced in E. coli as GST-tagged fusion protein (Santa Cruz Biotechnology). |
|---|
| Residue: | 317 |
|---|
| Sequence: | MFQAAERPQEWAMEGPRDGLKKERLLDDRHDSGLDSMKDEEYEQMVKELQEIRLEPQEVP
RGSEPWKQQLTEDGDSFLHLAIIHEEKALTMEVIRQVKGDLAFLNFQNNLQQTPLHLAVI
TNQPEIAEALLGAGCDPELRDFRGNTPLHLACEQGCLASVGVLTQSCTTPHLHSILKATN
YNGHTCLHLASIHGYLGIVELLVSLGADVNAQEPCNGRTALHLAVDLQNPDLVSLLLKCG
ADVNRVTYQGYSPYQLTWGRPSTRIQQQLGQLTLENLQMLPESEDEESYDTESEFTEFTE
DELPYDDCVFGGQRLTL
|
|
|
|---|
| BDBM50227494 |
|---|
| n/a |
|---|
| Name | BDBM50227494 |
|---|
| Synonyms: | CHEMBL257286 | N-(quinolin-8-yl)benzenesulfonamide | N-Quinolin-8-yl-benzenesulfonamide | cid_161167 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H12N2O2S |
|---|
| Mol. Mass. | 284.333 |
|---|
| SMILES | O=S(=O)(Nc1cccc2cccnc12)c1ccccc1 |
|---|
| Structure | 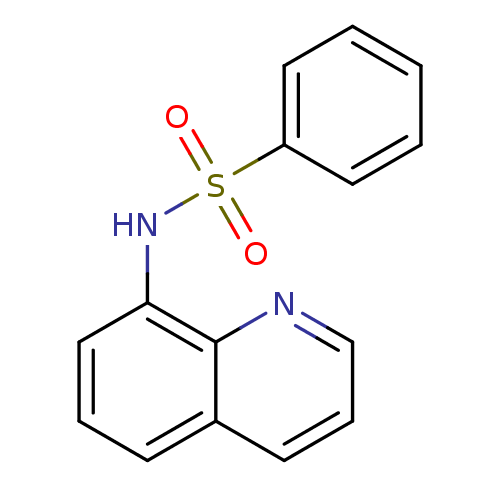
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xie, Y; Deng, S; Thomas, CJ; Liu, Y; Zhang, YQ; Rinderspacher, A; Huang, W; Gong, G; Wyler, M; Cayanis, E; Aulner, N; Többen, U; Chung, C; Pampou, S; Southall, N; Vidovic, D; Schürer, S; Branden, L; Davis, RE; Staudt, LM; Inglese, J; Austin, CP; Landry, DW; Smith, DH; Auld, DS Identification of N-(quinolin-8-yl)benzenesulfonamides as agents capable of down-regulating NFkappaB activity within two separate high-throughput screens of NFkappaB activation. Bioorg Med Chem Lett18:329-35 (2008) [PubMed] Article
Xie, Y; Deng, S; Thomas, CJ; Liu, Y; Zhang, YQ; Rinderspacher, A; Huang, W; Gong, G; Wyler, M; Cayanis, E; Aulner, N; Többen, U; Chung, C; Pampou, S; Southall, N; Vidovic, D; Schürer, S; Branden, L; Davis, RE; Staudt, LM; Inglese, J; Austin, CP; Landry, DW; Smith, DH; Auld, DS Identification of N-(quinolin-8-yl)benzenesulfonamides as agents capable of down-regulating NFkappaB activity within two separate high-throughput screens of NFkappaB activation. Bioorg Med Chem Lett18:329-35 (2008) [PubMed] Article