 Found 372 hits with Last Name = 'vidovic' and Initial = 'd'
Found 372 hits with Last Name = 'vidovic' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219573
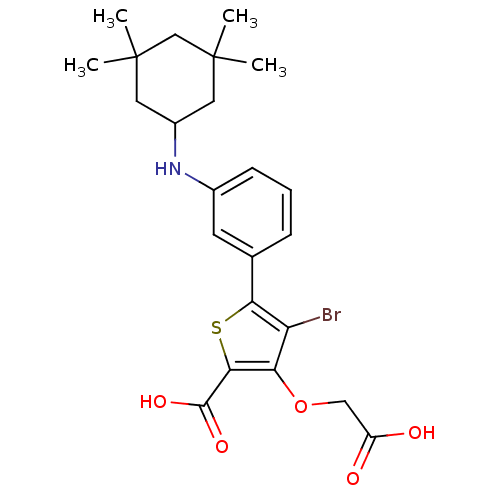
(4-BROMO-3-(CARBOXYMETHOXY)-5-{3-[(3,3,5,5-TETRAMET...)Show SMILES CC1(C)CC(CC(C)(C)C1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C23H28BrNO5S/c1-22(2)9-15(10-23(3,4)12-22)25-14-7-5-6-13(8-14)19-17(24)18(30-11-16(26)27)20(31-19)21(28)29/h5-8,15,25H,9-12H2,1-4H3,(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219573

(4-BROMO-3-(CARBOXYMETHOXY)-5-{3-[(3,3,5,5-TETRAMET...)Show SMILES CC1(C)CC(CC(C)(C)C1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C23H28BrNO5S/c1-22(2)9-15(10-23(3,4)12-22)25-14-7-5-6-13(8-14)19-17(24)18(30-11-16(26)27)20(31-19)21(28)29/h5-8,15,25H,9-12H2,1-4H3,(H,26,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14267
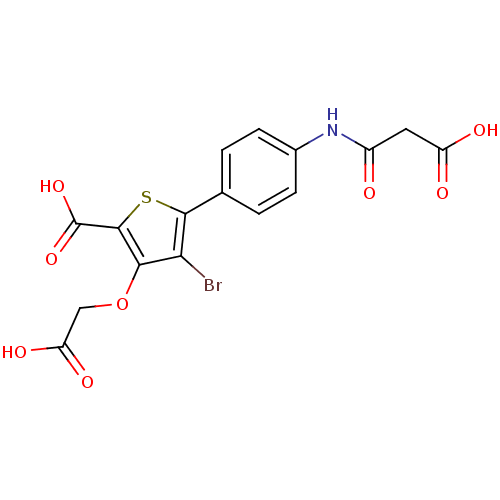
(4-bromo-3-(carboxymethoxy)-5-[4-(2-formamidoacetic...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1 Show InChI InChI=1S/C16H12BrNO8S/c17-12-13(26-6-11(22)23)15(16(24)25)27-14(12)7-1-3-8(4-2-7)18-9(19)5-10(20)21/h1-4H,5-6H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14267

(4-bromo-3-(carboxymethoxy)-5-[4-(2-formamidoacetic...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1 Show InChI InChI=1S/C16H12BrNO8S/c17-12-13(26-6-11(22)23)15(16(24)25)27-14(12)7-1-3-8(4-2-7)18-9(19)5-10(20)21/h1-4H,5-6H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118792
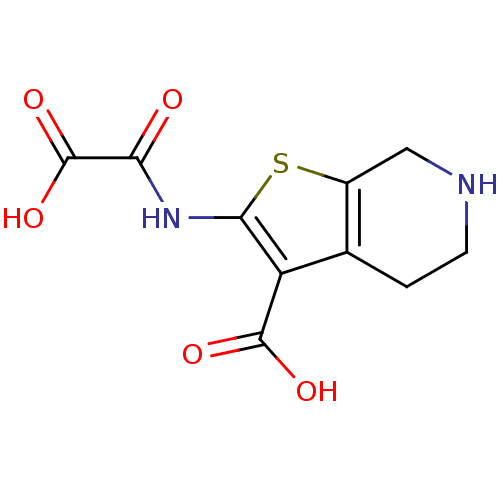
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50299461
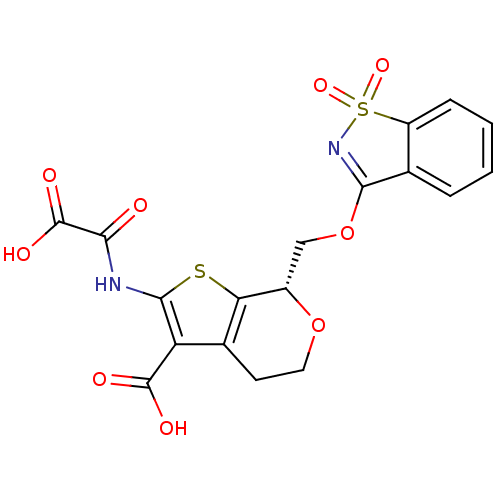
((S)-7-(1,1-Dioxo-1H-1lambda*6*-benzo[d]isothiazol-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](COC3=NS(=O)(=O)c4ccccc34)OCCc2c1C(O)=O |r,t:12| Show InChI InChI=1S/C18H14N2O9S2/c21-14(18(24)25)19-16-12(17(22)23)9-5-6-28-10(13(9)30-16)7-29-15-8-3-1-2-4-11(8)31(26,27)20-15/h1-4,10H,5-7H2,(H,19,21)(H,22,23)(H,24,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14258

(4-bromo-3-(carboxymethoxy)-5-[4-(pyridine-3-amido)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H13BrN2O6S/c20-14-15(28-9-13(23)24)17(19(26)27)29-16(14)10-3-5-12(6-4-10)22-18(25)11-2-1-7-21-8-11/h1-8H,9H2,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14258

(4-bromo-3-(carboxymethoxy)-5-[4-(pyridine-3-amido)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H13BrN2O6S/c20-14-15(28-9-13(23)24)17(19(26)27)29-16(14)10-3-5-12(6-4-10)22-18(25)11-2-1-7-21-8-11/h1-8H,9H2,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14255
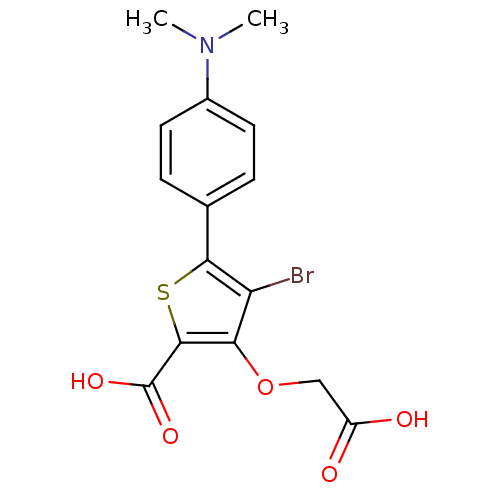
(4-bromo-3-(carboxymethoxy)-5-[4-(dimethylamino)phe...)Show SMILES CN(C)c1ccc(cc1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C15H14BrNO5S/c1-17(2)9-5-3-8(4-6-9)13-11(16)12(22-7-10(18)19)14(23-13)15(20)21/h3-6H,7H2,1-2H3,(H,18,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50299461

((S)-7-(1,1-Dioxo-1H-1lambda*6*-benzo[d]isothiazol-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](COC3=NS(=O)(=O)c4ccccc34)OCCc2c1C(O)=O |r,t:12| Show InChI InChI=1S/C18H14N2O9S2/c21-14(18(24)25)19-16-12(17(22)23)9-5-6-28-10(13(9)30-16)7-29-15-8-3-1-2-4-11(8)31(26,27)20-15/h1-4,10H,5-7H2,(H,19,21)(H,22,23)(H,24,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14245
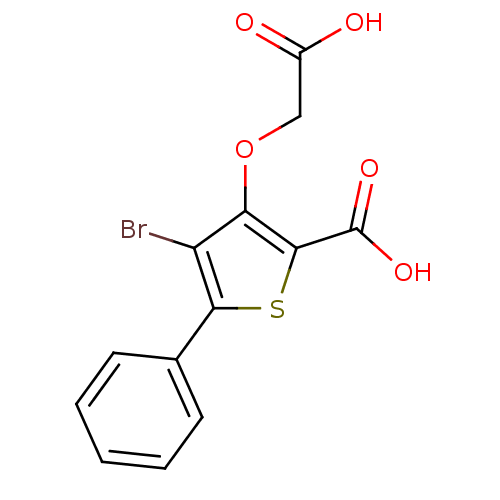
(4-bromo-3-(carboxymethoxy)-5-phenylthiophene-2-car...)Show InChI InChI=1S/C13H9BrO5S/c14-9-10(19-6-8(15)16)12(13(17)18)20-11(9)7-4-2-1-3-5-7/h1-5H,6H2,(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14255

(4-bromo-3-(carboxymethoxy)-5-[4-(dimethylamino)phe...)Show SMILES CN(C)c1ccc(cc1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C15H14BrNO5S/c1-17(2)9-5-3-8(4-6-9)13-11(16)12(22-7-10(18)19)14(23-13)15(20)21/h3-6H,7H2,1-2H3,(H,18,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14245

(4-bromo-3-(carboxymethoxy)-5-phenylthiophene-2-car...)Show InChI InChI=1S/C13H9BrO5S/c14-9-10(19-6-8(15)16)12(13(17)18)20-11(9)7-4-2-1-3-5-7/h1-5H,6H2,(H,15,16)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118751
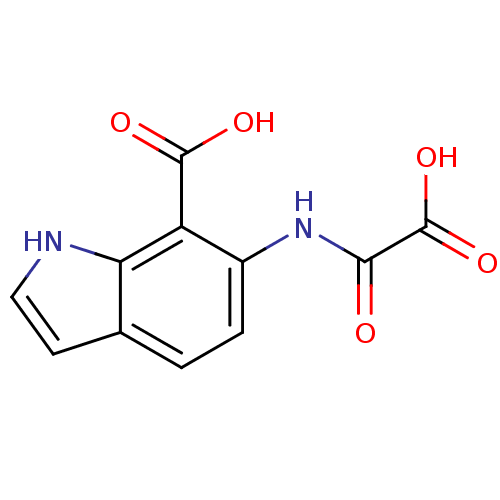
(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14230
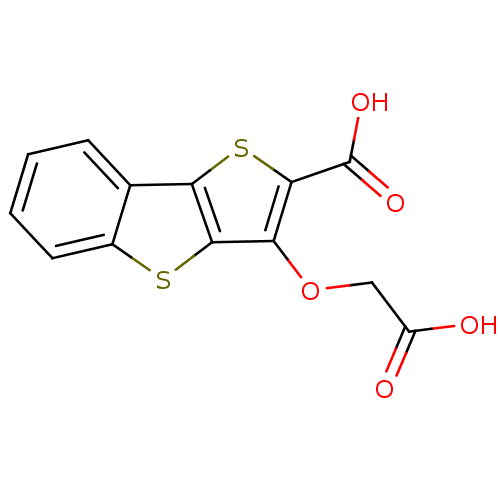
(5-(carboxymethoxy)-3,7-dithiatricyclo[6.4.0.0^{2,6...)Show InChI InChI=1S/C13H8O5S2/c14-8(15)5-18-9-11-10(20-12(9)13(16)17)6-3-1-2-4-7(6)19-11/h1-4H,5H2,(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN1 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118751

(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118762
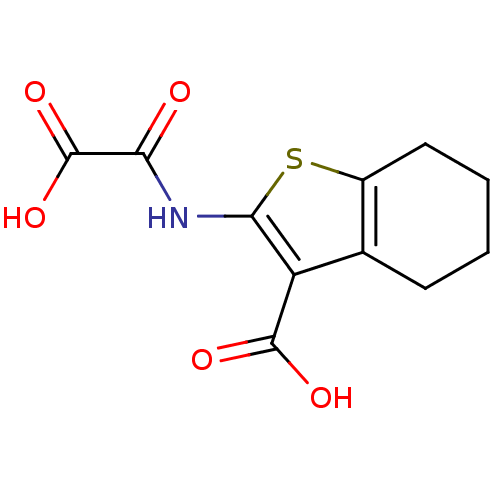
(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14230

(5-(carboxymethoxy)-3,7-dithiatricyclo[6.4.0.0^{2,6...)Show InChI InChI=1S/C13H8O5S2/c14-8(15)5-18-9-11-10(20-12(9)13(16)17)6-3-1-2-4-7(6)19-11/h1-4H,5H2,(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50299462

(2-(3-(dihydroxymethyl)naphthalen-2-ylamino)-2-oxoa...)Show InChI InChI=1S/C13H11NO5/c15-11(13(18)19)14-10-6-8-4-2-1-3-7(8)5-9(10)12(16)17/h1-6,12,16-17H,(H,14,15)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118744
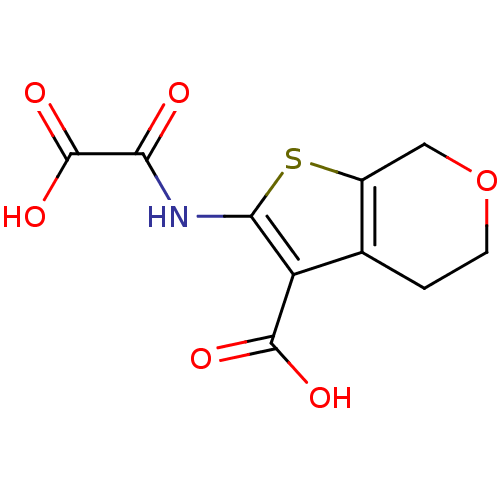
(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...)Show InChI InChI=1S/C10H9NO6S/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118744

(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...)Show InChI InChI=1S/C10H9NO6S/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118778

(5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908)Show InChI InChI=1S/C9H6INO5/c10-4-1-2-6(5(3-4)8(13)14)11-7(12)9(15)16/h1-3H,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50299462

(2-(3-(dihydroxymethyl)naphthalen-2-ylamino)-2-oxoa...)Show InChI InChI=1S/C13H11NO5/c15-11(13(18)19)14-10-6-8-4-2-1-3-7(8)5-9(10)12(16)17/h1-6,12,16-17H,(H,14,15)(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118778

(5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908)Show InChI InChI=1S/C9H6INO5/c10-4-1-2-6(5(3-4)8(13)14)11-7(12)9(15)16/h1-3H,(H,11,12)(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118749
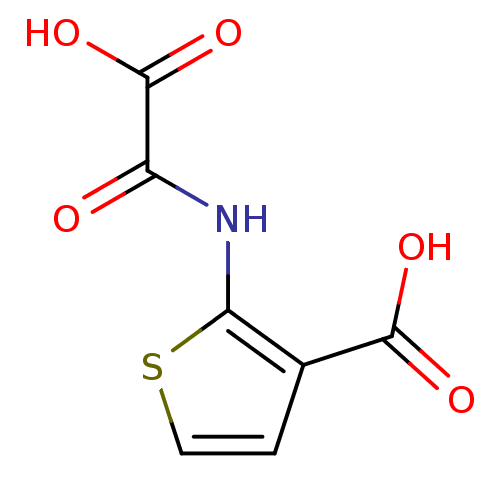
(2-(Oxalyl-amino)-thiophene-3-carboxylic acid | 2-(...)Show InChI InChI=1S/C7H5NO5S/c9-4(7(12)13)8-5-3(6(10)11)1-2-14-5/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118762

(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118751

(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon
(Homo sapiens (Human)) | BDBM50118744

(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...)Show InChI InChI=1S/C10H9NO6S/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50299461

((S)-7-(1,1-Dioxo-1H-1lambda*6*-benzo[d]isothiazol-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](COC3=NS(=O)(=O)c4ccccc34)OCCc2c1C(O)=O |r,t:12| Show InChI InChI=1S/C18H14N2O9S2/c21-14(18(24)25)19-16-12(17(22)23)9-5-6-28-10(13(9)30-16)7-29-15-8-3-1-2-4-11(8)31(26,27)20-15/h1-4,10H,5-7H2,(H,19,21)(H,22,23)(H,24,25)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118789
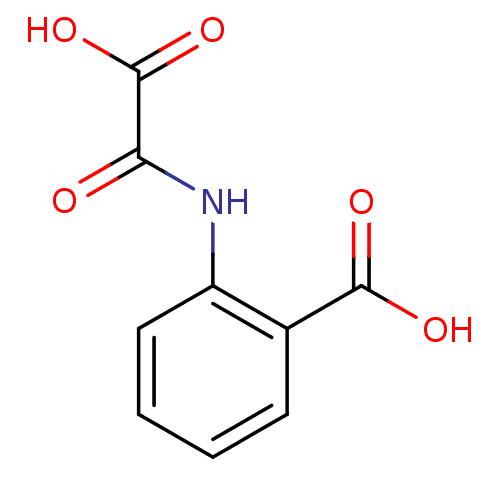
(2-(oxaloamino)benzoic acid | CHEMBL139050)Show InChI InChI=1S/C9H7NO5/c11-7(9(14)15)10-6-4-2-1-3-5(6)8(12)13/h1-4H,(H,10,11)(H,12,13)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118751

(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon
(Homo sapiens (Human)) | BDBM50118778

(5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908)Show InChI InChI=1S/C9H6INO5/c10-4-1-2-6(5(3-4)8(13)14)11-7(12)9(15)16/h1-3H,(H,11,12)(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon
(Homo sapiens (Human)) | BDBM50118751

(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM14267

(4-bromo-3-(carboxymethoxy)-5-[4-(2-formamidoacetic...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1 Show InChI InChI=1S/C16H12BrNO8S/c17-12-13(26-6-11(22)23)15(16(24)25)27-14(12)7-1-3-8(4-2-7)18-9(19)5-10(20)21/h1-4H,5-6H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50118789

(2-(oxaloamino)benzoic acid | CHEMBL139050)Show InChI InChI=1S/C9H7NO5/c11-7(9(14)15)10-6-4-2-1-3-5(6)8(12)13/h1-4H,(H,10,11)(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50299462

(2-(3-(dihydroxymethyl)naphthalen-2-ylamino)-2-oxoa...)Show InChI InChI=1S/C13H11NO5/c15-11(13(18)19)14-10-6-8-4-2-1-3-7(8)5-9(10)12(16)17/h1-6,12,16-17H,(H,14,15)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118762

(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon
(Homo sapiens (Human)) | BDBM50299462

(2-(3-(dihydroxymethyl)naphthalen-2-ylamino)-2-oxoa...)Show InChI InChI=1S/C13H11NO5/c15-11(13(18)19)14-10-6-8-4-2-1-3-7(8)5-9(10)12(16)17/h1-6,12,16-17H,(H,14,15)(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon
(Homo sapiens (Human)) | BDBM50118762

(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118778

(5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908)Show InChI InChI=1S/C9H6INO5/c10-4-1-2-6(5(3-4)8(13)14)11-7(12)9(15)16/h1-3H,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118744

(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...)Show InChI InChI=1S/C10H9NO6S/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM14258

(4-bromo-3-(carboxymethoxy)-5-[4-(pyridine-3-amido)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H13BrN2O6S/c20-14-15(28-9-13(23)24)17(19(26)27)29-16(14)10-3-5-12(6-4-10)22-18(25)11-2-1-7-21-8-11/h1-8H,9H2,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118779
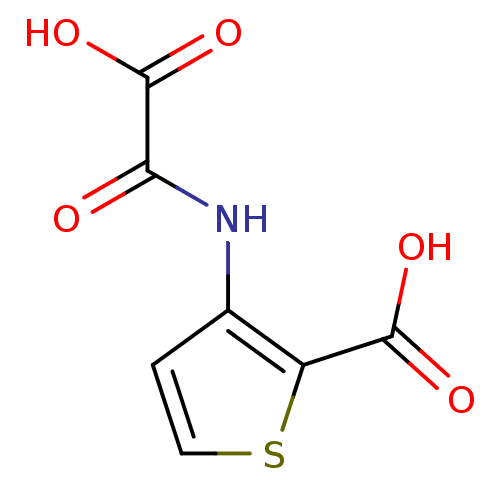
(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118792

(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118749

(2-(Oxalyl-amino)-thiophene-3-carboxylic acid | 2-(...)Show InChI InChI=1S/C7H5NO5S/c9-4(7(12)13)8-5-3(6(10)11)1-2-14-5/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118749

(2-(Oxalyl-amino)-thiophene-3-carboxylic acid | 2-(...)Show InChI InChI=1S/C7H5NO5S/c9-4(7(12)13)8-5-3(6(10)11)1-2-14-5/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data