

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Kappa-type opioid receptor | ||
| Ligand | BDBM50271708 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_556764 (CHEMBL961462) | ||
| IC50 | 365±n/a nM | ||
| Citation |  Martínez-Mayorga, K; Medina-Franco, JL; Giulianotti, MA; Pinilla, C; Dooley, CT; Appel, JR; Houghten, RA Conformation-opioid activity relationships of bicyclic guanidines from 3D similarity analysis. Bioorg Med Chem16:5932-8 (2008) [PubMed] Article Martínez-Mayorga, K; Medina-Franco, JL; Giulianotti, MA; Pinilla, C; Dooley, CT; Appel, JR; Houghten, RA Conformation-opioid activity relationships of bicyclic guanidines from 3D similarity analysis. Bioorg Med Chem16:5932-8 (2008) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Kappa-type opioid receptor | |||
| Name: | Kappa-type opioid receptor | ||
| Synonyms: | Kappa Opioid Receptor | OPIATE Kappa | OPRK1 | OPRK_CAVPO | Opiate Kappa 1 | mu/kappa opioid receptor | ||
| Type: | G Protein-Coupled Receptor (GPCR) | ||
| Mol. Mass.: | 42744.99 | ||
| Organism: | Cavia porcellus (domestic guinea pig) | ||
| Description: | P41144 | ||
| Residue: | 380 | ||
| Sequence: |
| ||
| BDBM50271708 | |||
| n/a | |||
| Name | BDBM50271708 | ||
| Synonyms: | (2S,5R)-2,5-Dicyclohexyl-1-(4-methyl-cyclohexylmethyl)-2,3,5,6-tetrahydro-1H-imidazo[1,2-a]imidazole | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H44N3 | ||
| Mol. Mass. | 386.6364 | ||
| SMILES | CC1CCC(CN2[C@H](CN3[C@@H](C[NH+]=C23)C2CCCCC2)C2CCCCC2)CC1 |r,wU:10.15,wD:7.22,t:12,(10.65,-14.86,;11.71,-13.74,;11.28,-12.26,;12.34,-11.15,;13.83,-11.52,;14.89,-10.4,;14.46,-8.92,;15.39,-7.69,;14.52,-6.42,;13.05,-6.86,;11.59,-6.34,;10.65,-7.57,;11.52,-8.84,;13,-8.41,;11.15,-4.87,;12.21,-3.76,;11.77,-2.29,;10.28,-1.92,;9.21,-3.04,;9.65,-4.52,;16.93,-7.73,;17.67,-9.1,;19.2,-9.14,;20.01,-7.83,;19.28,-6.48,;17.73,-6.43,;14.28,-12.99,;13.21,-14.11,)| | ||
| Structure | 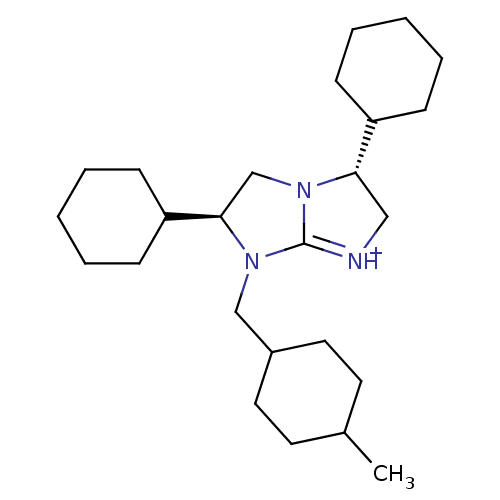
| ||