| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingosine 1-phosphate receptor 3 |
|---|
| Ligand | BDBM50419969 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_809730 (CHEMBL2016149) |
|---|
| EC50 | >10000±n/a nM |
|---|
| Citation |  Xu, H; Zhang, H; Luan, L; Xu, Y; Li, C; Wang, Y; Han, F; Yang, T; Ren, F; Xiang, JN; Elliott, JD; Zhao, Y; Guo, TB; Lu, H; Zhang, W; Hirst, D; Lindon, M; Lin, X Discovery of thiadiazole amides as potent, S1P3-sparing agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett22:2456-9 (2012) [PubMed] Article Xu, H; Zhang, H; Luan, L; Xu, Y; Li, C; Wang, Y; Han, F; Yang, T; Ren, F; Xiang, JN; Elliott, JD; Zhao, Y; Guo, TB; Lu, H; Zhang, W; Hirst, D; Lindon, M; Lin, X Discovery of thiadiazole amides as potent, S1P3-sparing agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett22:2456-9 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingosine 1-phosphate receptor 3 |
|---|
| Name: | Sphingosine 1-phosphate receptor 3 |
|---|
| Synonyms: | C9orf108 | C9orf47 | EDG3 | Endothelial differentiation G-protein coupled receptor 3 | S1P receptor 3 | S1P receptor Edg-3 | S1P3 | S1PR3 | S1PR3_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor 3 (S1P3) | Sphingosine 1-phosphate receptor Edg-3 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 42278.13 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q99500 |
|---|
| Residue: | 378 |
|---|
| Sequence: | MATALPPRLQPVRGNETLREHYQYVGKLAGRLKEASEGSTLTTVLFLVICSFIVLENLMV
LIAIWKNNKFHNRMYFFIGNLALCDLLAGIAYKVNILMSGKKTFSLSPTVWFLREGSMFV
ALGASTCSLLAIAIERHLTMIKMRPYDANKRHRVFLLIGMCWLIAFTLGALPILGWNCLH
NLPDCSTILPLYSKKYIAFCISIFTAILVTIVILYARIYFLVKSSSRKVANHNNSERSMA
LLRTVVIVVSVFIACWSPLFILFLIDVACRVQACPILFKAQWFIVLAVLNSAMNPVIYTL
ASKEMRRAFFRLVCNCLVRGRGARASPIQPALDPSRSKSSSSNNSSHSPKVKEDLPHTAP
SSCIMDKNAALQNGIFCN
|
|
|
|---|
| BDBM50419969 |
|---|
| n/a |
|---|
| Name | BDBM50419969 |
|---|
| Synonyms: | CHEMBL2011743 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H19F3N4OS |
|---|
| Mol. Mass. | 444.473 |
|---|
| SMILES | CCCCN(C(=O)c1ccc(cc1)C(F)(F)F)c1nnc(s1)-c1ccc2cc[nH]c2c1 |
|---|
| Structure | 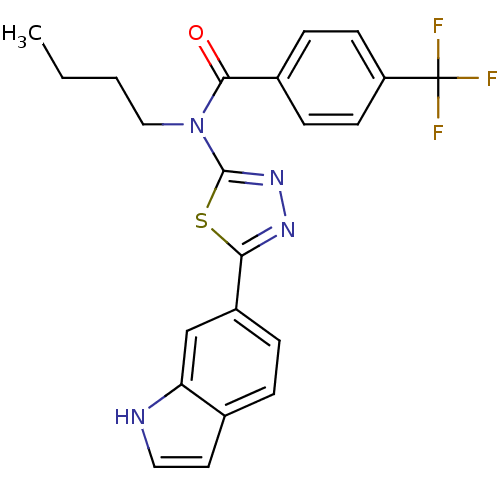
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xu, H; Zhang, H; Luan, L; Xu, Y; Li, C; Wang, Y; Han, F; Yang, T; Ren, F; Xiang, JN; Elliott, JD; Zhao, Y; Guo, TB; Lu, H; Zhang, W; Hirst, D; Lindon, M; Lin, X Discovery of thiadiazole amides as potent, S1P3-sparing agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett22:2456-9 (2012) [PubMed] Article
Xu, H; Zhang, H; Luan, L; Xu, Y; Li, C; Wang, Y; Han, F; Yang, T; Ren, F; Xiang, JN; Elliott, JD; Zhao, Y; Guo, TB; Lu, H; Zhang, W; Hirst, D; Lindon, M; Lin, X Discovery of thiadiazole amides as potent, S1P3-sparing agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett22:2456-9 (2012) [PubMed] Article