

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Tyrosine-protein kinase receptor UFO | ||
| Ligand | BDBM50384581 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_820660 (CHEMBL2037931) | ||
| IC50 | 12±n/a nM | ||
| Citation |  Liu, J; Yang, C; Simpson, C; Deryckere, D; Van Deusen, A; Miley, MJ; Kireev, D; Norris-Drouin, J; Sather, S; Hunter, D; Korboukh, VK; Patel, HS; Janzen, WP; Machius, M; Johnson, GL; Earp, HS; Graham, DK; Frye, SV; Wang, X Discovery of Novel Small Molecule Mer Kinase Inhibitors for the Treatment of Pediatric Acute Lymphoblastic Leukemia. ACS Med Chem Lett3:129-134 (2012) [PubMed] Article Liu, J; Yang, C; Simpson, C; Deryckere, D; Van Deusen, A; Miley, MJ; Kireev, D; Norris-Drouin, J; Sather, S; Hunter, D; Korboukh, VK; Patel, HS; Janzen, WP; Machius, M; Johnson, GL; Earp, HS; Graham, DK; Frye, SV; Wang, X Discovery of Novel Small Molecule Mer Kinase Inhibitors for the Treatment of Pediatric Acute Lymphoblastic Leukemia. ACS Med Chem Lett3:129-134 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Tyrosine-protein kinase receptor UFO | |||
| Name: | Tyrosine-protein kinase receptor UFO | ||
| Synonyms: | AXL | AXL oncogene | TEL/AXL | Tyrosine-protein kinase receptor UFO (AXL) | UFO | UFO_HUMAN | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 98316.97 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P30530 | ||
| Residue: | 894 | ||
| Sequence: |
| ||
| BDBM50384581 | |||
| n/a | |||
| Name | BDBM50384581 | ||
| Synonyms: | CHEMBL2036804 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H37N9 | ||
| Mol. Mass. | 463.6216 | ||
| SMILES | CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(nc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(-8.83,-18.78,;-8.83,-20.32,;-7.5,-21.09,;-7.5,-22.63,;-6.17,-23.41,;-4.83,-22.64,;-4.83,-21.09,;-3.5,-20.32,;-2.17,-21.08,;-.69,-20.61,;.22,-21.86,;-.69,-23.12,;-.22,-24.58,;-1.25,-25.72,;-.77,-27.18,;-1.81,-28.32,;-3.31,-28,;-4.35,-29.14,;-3.78,-26.53,;-2.76,-25.39,;-2.17,-22.64,;-3.5,-23.41,;-.22,-19.14,;-1.25,-18.01,;-.78,-16.54,;.73,-16.22,;1.77,-17.36,;1.29,-18.82,;1.2,-14.75,;2.71,-14.44,;3.19,-12.98,;2.16,-11.83,;.65,-12.15,;.17,-13.62,)| | ||
| Structure | 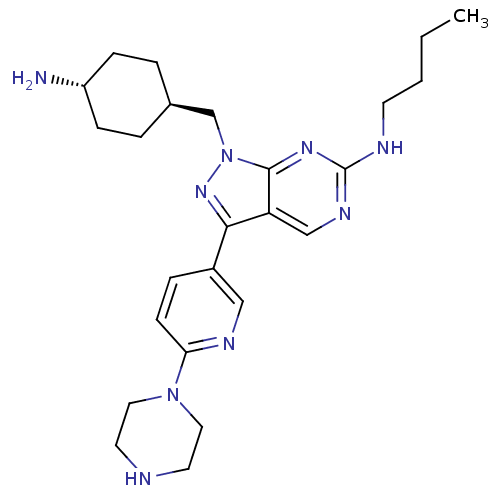
| ||