| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase Lck |
|---|
| Ligand | BDBM50386749 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_827009 (CHEMBL2050073) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Li, WW; Wang, XY; Zheng, RL; Yan, HX; Cao, ZX; Zhong, L; Wang, ZR; Ji, P; Yang, LL; Wang, LJ; Xu, Y; Liu, JJ; Yang, J; Zhang, CH; Ma, S; Feng, S; Sun, QZ; Wei, YQ; Yang, SY Discovery of the novel potent and selective FLT3 inhibitor 1-{5-[7-(3- morpholinopropoxy)quinazolin-4-ylthio]-[1,3,4]thiadiazol-2-yl}-3-p-tolylurea and its anti-acute myeloid leukemia (AML) activities in vitro and in vivo. J Med Chem55:3852-66 (2012) [PubMed] Article Li, WW; Wang, XY; Zheng, RL; Yan, HX; Cao, ZX; Zhong, L; Wang, ZR; Ji, P; Yang, LL; Wang, LJ; Xu, Y; Liu, JJ; Yang, J; Zhang, CH; Ma, S; Feng, S; Sun, QZ; Wei, YQ; Yang, SY Discovery of the novel potent and selective FLT3 inhibitor 1-{5-[7-(3- morpholinopropoxy)quinazolin-4-ylthio]-[1,3,4]thiadiazol-2-yl}-3-p-tolylurea and its anti-acute myeloid leukemia (AML) activities in vitro and in vivo. J Med Chem55:3852-66 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase Lck |
|---|
| Name: | Tyrosine-protein kinase Lck |
|---|
| Synonyms: | 2.7.10.2 | LCK | LCK_HUMAN | LSK | Leukocyte C-terminal Src kinase | Lymphocyte cell-specific protein-tyrosine kinase | Lymphocyte-specific protein tyrosine kinase | P56-LCK | Protein YT16 | Proto-oncogene Lck | Proto-oncogene tyrosine-protein kinase LCK | Src/Lck kinase | T cell-specific protein-tyrosine kinase |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 57987.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06239 |
|---|
| Residue: | 509 |
|---|
| Sequence: | MGCGCSSHPEDDWMENIDVCENCHYPIVPLDGKGTLLIRNGSEVRDPLVTYEGSNPPASP
LQDNLVIALHSYEPSHDGDLGFEKGEQLRILEQSGEWWKAQSLTTGQEGFIPFNFVAKAN
SLEPEPWFFKNLSRKDAERQLLAPGNTHGSFLIRESESTAGSFSLSVRDFDQNQGEVVKH
YKIRNLDNGGFYISPRITFPGLHELVRHYTNASDGLCTRLSRPCQTQKPQKPWWEDEWEV
PRETLKLVERLGAGQFGEVWMGYYNGHTKVAVKSLKQGSMSPDAFLAEANLMKQLQHQRL
VRLYAVVTQEPIYIITEYMENGSLVDFLKTPSGIKLTINKLLDMAAQIAEGMAFIEERNY
IHRDLRAANILVSDTLSCKIADFGLARLIEDNEYTAREGAKFPIKWTAPEAINYGTFTIK
SDVWSFGILLTEIVTHGRIPYPGMTNPEVIQNLERGYRMVRPDNCPEELYQLMRLCWKER
PEDRPTFDYLRSVLEDFFTATEGQYQPQP
|
|
|
|---|
| BDBM50386749 |
|---|
| n/a |
|---|
| Name | BDBM50386749 |
|---|
| Synonyms: | CHEMBL2046883 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H27N7O3S2 |
|---|
| Mol. Mass. | 537.657 |
|---|
| SMILES | Cc1ccc(NC(=O)Nc2nnc(Sc3ncnc4cc(OCCCN5CCOCC5)ccc34)s2)cc1 |
|---|
| Structure | 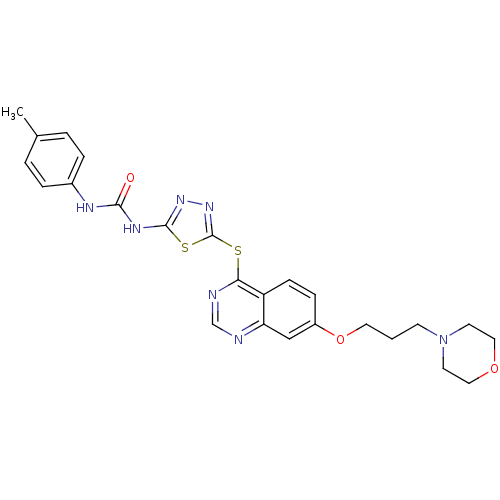
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, WW; Wang, XY; Zheng, RL; Yan, HX; Cao, ZX; Zhong, L; Wang, ZR; Ji, P; Yang, LL; Wang, LJ; Xu, Y; Liu, JJ; Yang, J; Zhang, CH; Ma, S; Feng, S; Sun, QZ; Wei, YQ; Yang, SY Discovery of the novel potent and selective FLT3 inhibitor 1-{5-[7-(3- morpholinopropoxy)quinazolin-4-ylthio]-[1,3,4]thiadiazol-2-yl}-3-p-tolylurea and its anti-acute myeloid leukemia (AML) activities in vitro and in vivo. J Med Chem55:3852-66 (2012) [PubMed] Article
Li, WW; Wang, XY; Zheng, RL; Yan, HX; Cao, ZX; Zhong, L; Wang, ZR; Ji, P; Yang, LL; Wang, LJ; Xu, Y; Liu, JJ; Yang, J; Zhang, CH; Ma, S; Feng, S; Sun, QZ; Wei, YQ; Yang, SY Discovery of the novel potent and selective FLT3 inhibitor 1-{5-[7-(3- morpholinopropoxy)quinazolin-4-ylthio]-[1,3,4]thiadiazol-2-yl}-3-p-tolylurea and its anti-acute myeloid leukemia (AML) activities in vitro and in vivo. J Med Chem55:3852-66 (2012) [PubMed] Article