 Found 2099 hits with Last Name = 'feng' and Initial = 's'
Found 2099 hits with Last Name = 'feng' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569138
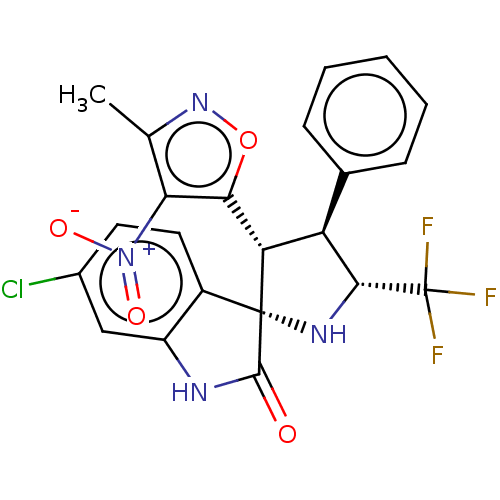
(CHEMBL4871070)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Cl)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569140

(CHEMBL4863792)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Br)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM31197
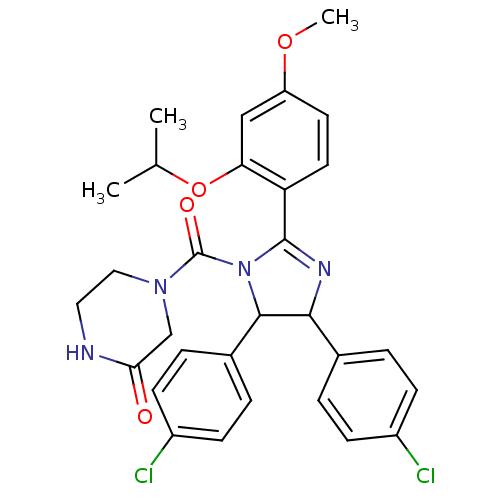
(CHEMBL211045 | Nutlin-3 | med.21724, Compound 186)Show SMILES COc1ccc(C2=NC(C(N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569136

(CHEMBL4874111)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(F)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569137

(CHEMBL4860936)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Cl)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569139
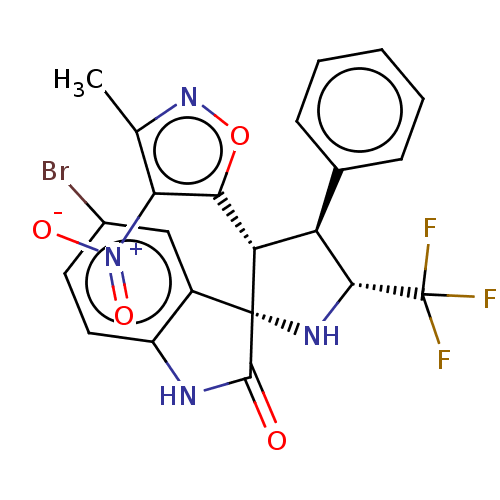
(CHEMBL4863780)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Br)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569144

(CHEMBL4861981)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(F)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569143

(CHEMBL4876458)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc(F)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569158

(CHEMBL4856044)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccco2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569135
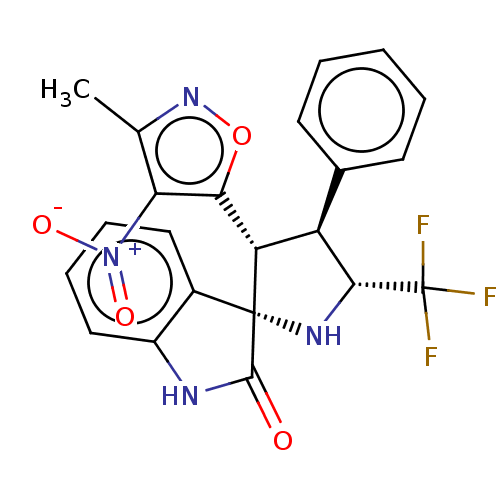
(CHEMBL4874952)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569159
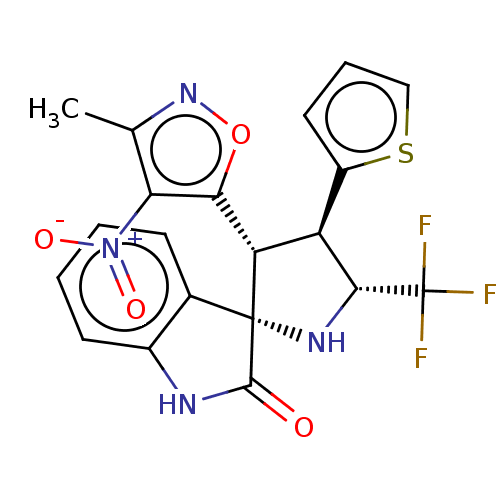
(CHEMBL4870214)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccs2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569142
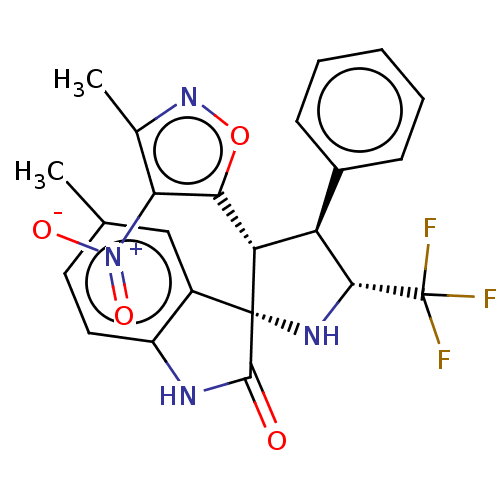
(CHEMBL4877534)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(C)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569152
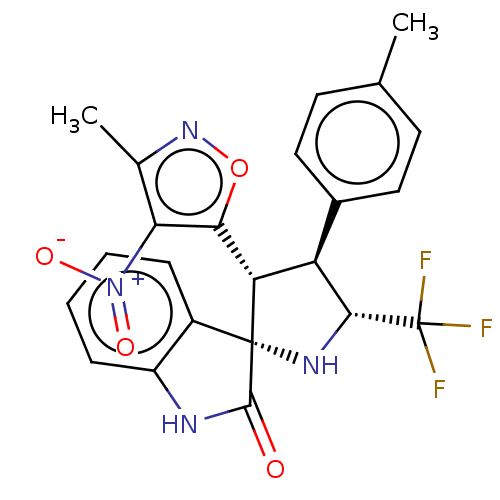
(CHEMBL4859985)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(C)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569149
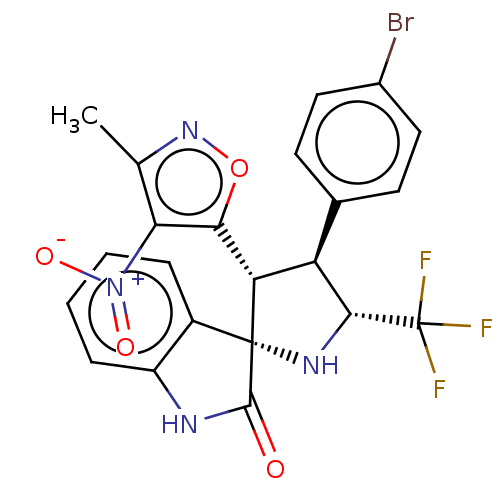
(CHEMBL4877092)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Br)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569154
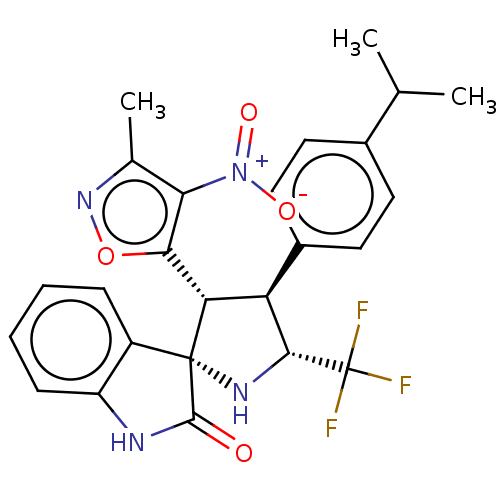
(CHEMBL4860559)Show SMILES CC(C)c1ccc(cc1)[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569156

(CHEMBL4878727)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Cl)c(Cl)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569146
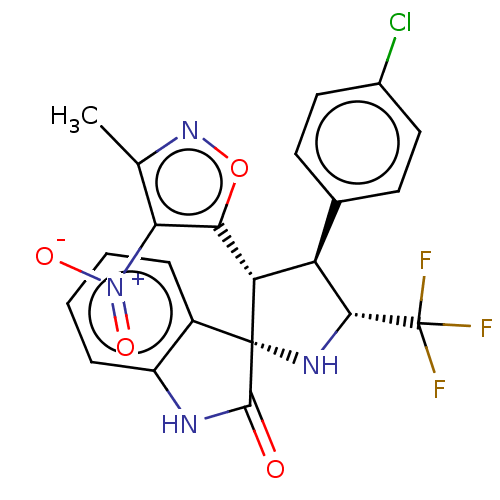
(CHEMBL4870252)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Cl)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569148
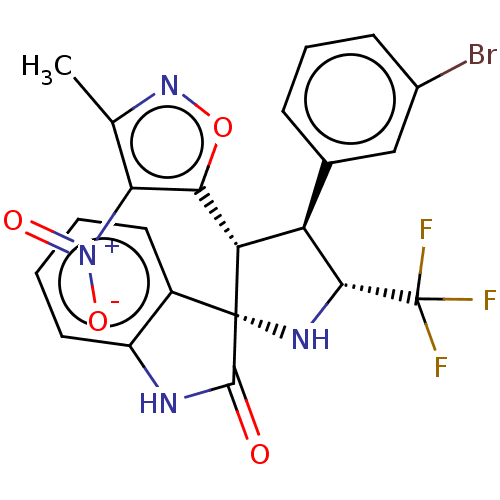
(CHEMBL4848056)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc(Br)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569141

(CHEMBL4878828)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(cc23)[N+]([O-])=O)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569157
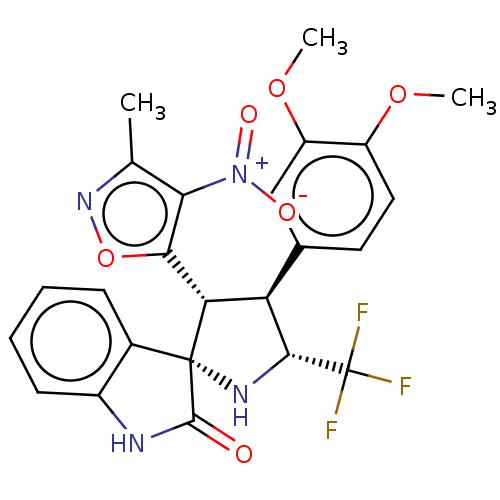
(CHEMBL4855468)Show SMILES COc1ccc(cc1OC)[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569164
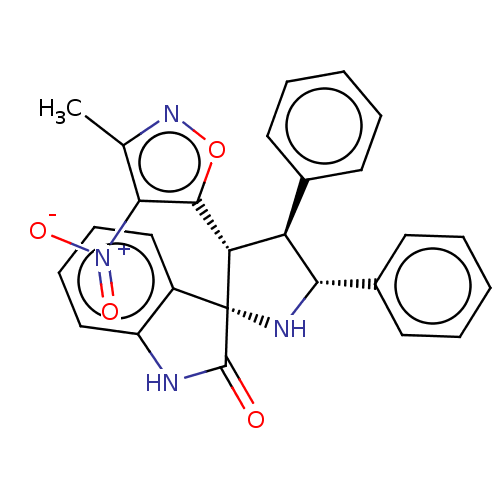
(CHEMBL4874048)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)c2ccccc2)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569150

(CHEMBL4859002)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(cc2)[N+]([O-])=O)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569160
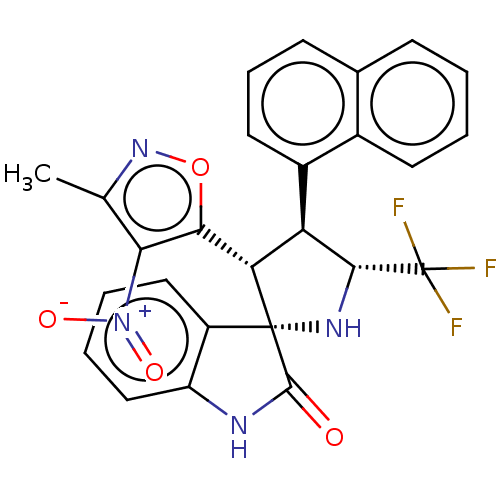
(CHEMBL4848654)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc3ccccc23)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569153

(CHEMBL4852407)Show SMILES COc1ccccc1[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569147

(CHEMBL4860063)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2Br)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569155
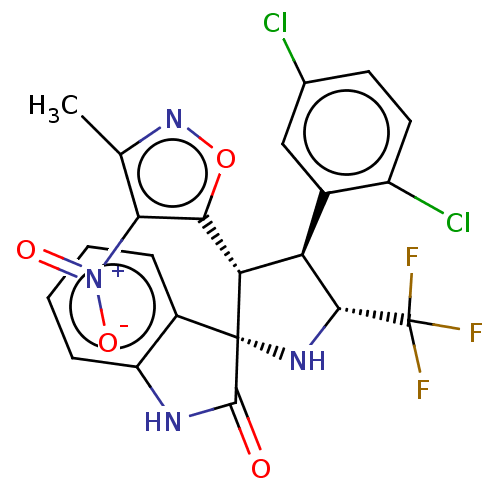
(CHEMBL4869937)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cc(Cl)ccc2Cl)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569161

(CHEMBL4849608)Show SMILES CN1C(=O)[C@@]2(N[C@H]([C@@H]([C@H]2c2onc(C)c2[N+]([O-])=O)c2ccccc2)C(F)(F)F)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569162
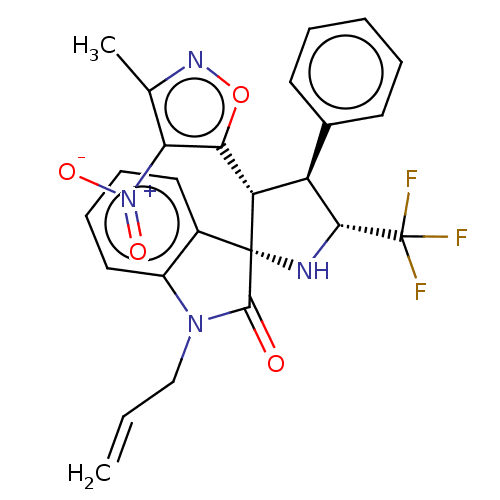
(CHEMBL4849387)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)N(CC=C)c3ccccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569163

(CHEMBL4874032)Show SMILES CC(=O)N1C(=O)[C@@]2(N[C@H]([C@@H]([C@H]2c2onc(C)c2[N+]([O-])=O)c2ccccc2)C(F)(F)F)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569145

(CHEMBL4864855)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2Cl)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569151
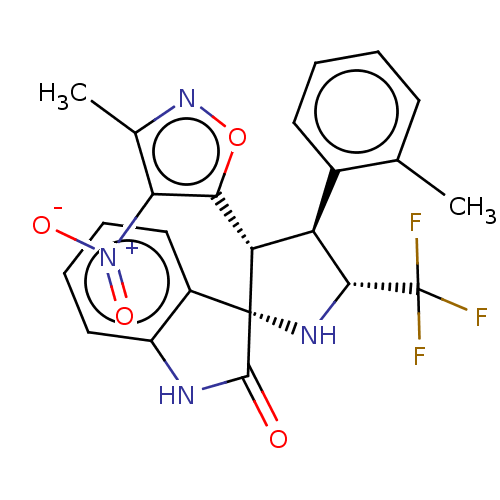
(CHEMBL4873310)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2C)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628884
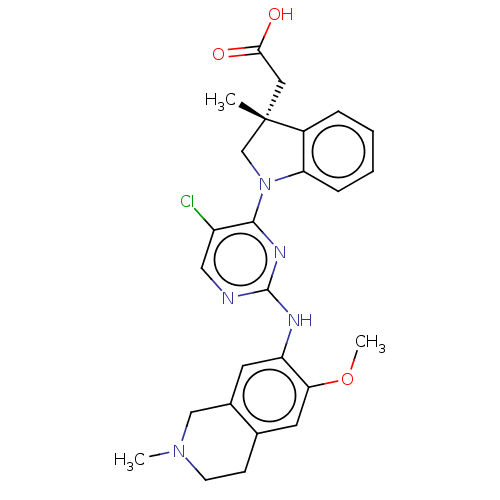
(US20230339896, Example 2)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1C[C@](C)(CC(O)=O)c2ccccc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628887
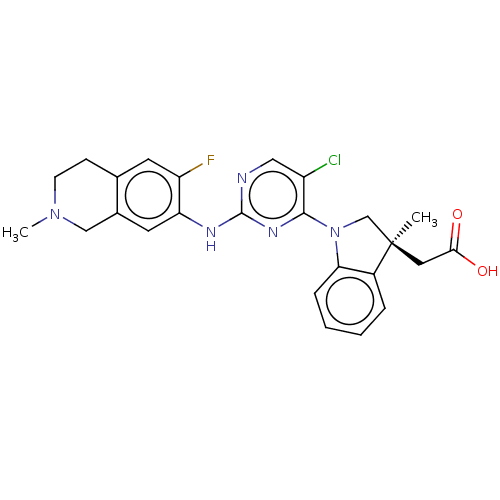
(US20230339896, Example 5)Show SMILES CN1CCc2cc(F)c(Nc3ncc(Cl)c(n3)N3C[C@@](C)(CC(O)=O)c4ccccc34)cc2C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628883

(US20230339896, Example 1)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1CC(C)(CC(O)=O)c2ccccc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628891

(US20230339896, Example 10)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1CC(C)(CC(O)=O)c2ccc(F)cc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628886
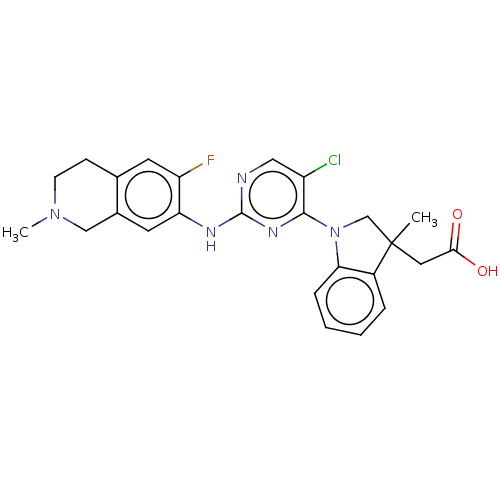
(US20230339896, Example 4)Show SMILES CN1CCc2cc(F)c(Nc3ncc(Cl)c(n3)N3CC(C)(CC(O)=O)c4ccccc34)cc2C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628888
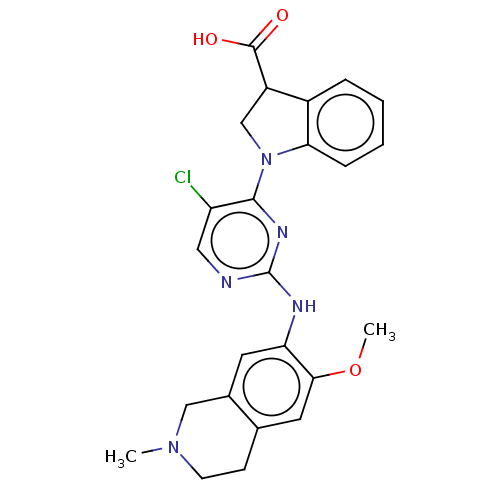
(US20230339896, Example 7)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1CC(C(O)=O)c2ccccc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50336375
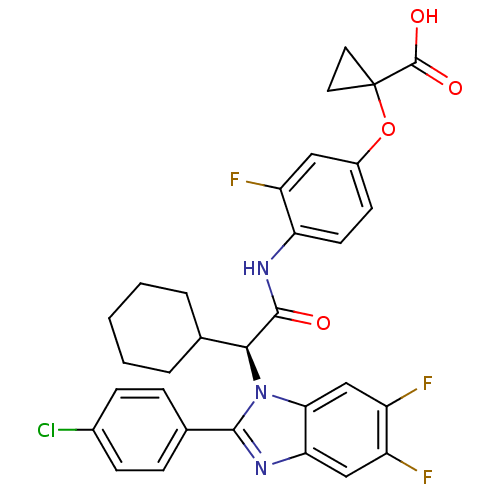
((S)-1-(4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-be...)Show SMILES OC(=O)C1(CC1)Oc1ccc(NC(=O)[C@H](C2CCCCC2)n2c(nc3cc(F)c(F)cc23)-c2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C31H27ClF3N3O4/c32-19-8-6-18(7-9-19)28-36-25-15-21(33)22(34)16-26(25)38(28)27(17-4-2-1-3-5-17)29(39)37-24-11-10-20(14-23(24)35)42-31(12-13-31)30(40)41/h6-11,14-17,27H,1-5,12-13H2,(H,37,39)(H,40,41)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human FXR by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1134-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.123
BindingDB Entry DOI: 10.7270/Q2MS3T1N |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50429477
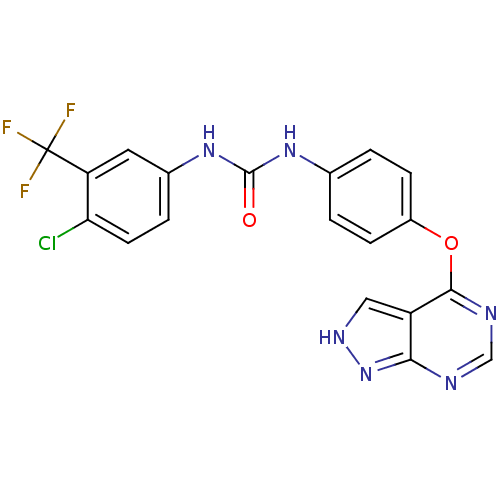
(CHEMBL2332840)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ncnc4n[nH]cc34)cc2)ccc1Cl Show InChI InChI=1S/C19H12ClF3N6O2/c20-15-6-3-11(7-14(15)19(21,22)23)28-18(30)27-10-1-4-12(5-2-10)31-17-13-8-26-29-16(13)24-9-25-17/h1-9H,(H2,27,28,30)(H,24,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 in human MV4-11 cells assessed as inhibition of Erk1/2 phosphorylation after 20 hrs by Western blotting analysis |
J Med Chem 56: 1641-55 (2013)
Article DOI: 10.1021/jm301537p
BindingDB Entry DOI: 10.7270/Q29S1SC5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628890
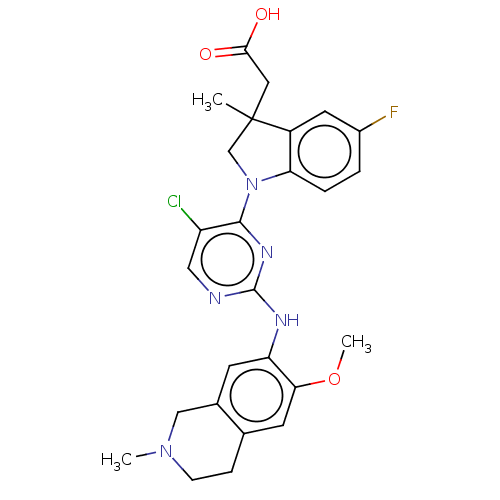
(US20230339896, Example 9)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1CC(C)(CC(O)=O)c2cc(F)ccc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM628889
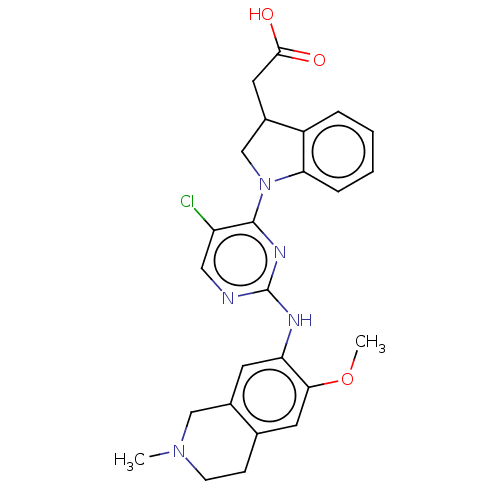
(US20230339896, Example 8)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(Cl)c(n1)N1CC(CC(O)=O)c2ccccc12 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50429477

(CHEMBL2332840)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ncnc4n[nH]cc34)cc2)ccc1Cl Show InChI InChI=1S/C19H12ClF3N6O2/c20-15-6-3-11(7-14(15)19(21,22)23)28-18(30)27-10-1-4-12(5-2-10)31-17-13-8-26-29-16(13)24-9-25-17/h1-9H,(H2,27,28,30)(H,24,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 in human MV4-11 cells assessed as inhibition of STAT5 phosphorylation after 20 hrs by Western blotting analysis |
J Med Chem 56: 1641-55 (2013)
Article DOI: 10.1021/jm301537p
BindingDB Entry DOI: 10.7270/Q29S1SC5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50429477

(CHEMBL2332840)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ncnc4n[nH]cc34)cc2)ccc1Cl Show InChI InChI=1S/C19H12ClF3N6O2/c20-15-6-3-11(7-14(15)19(21,22)23)28-18(30)27-10-1-4-12(5-2-10)31-17-13-8-26-29-16(13)24-9-25-17/h1-9H,(H2,27,28,30)(H,24,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 phosphorylation in human MV4-11 cells after 20 hrs by Western blotting analysis |
J Med Chem 56: 1641-55 (2013)
Article DOI: 10.1021/jm301537p
BindingDB Entry DOI: 10.7270/Q29S1SC5 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50336376
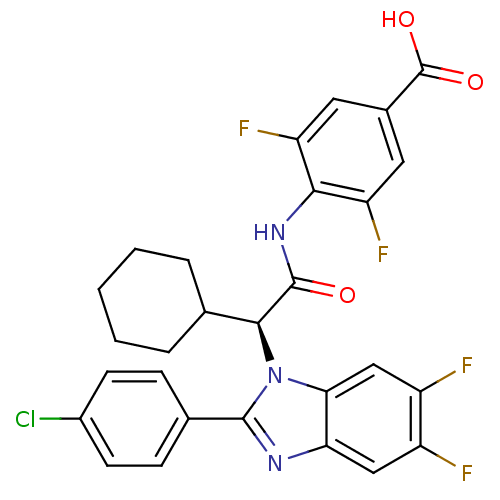
((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...)Show SMILES OC(=O)c1cc(F)c(NC(=O)[C@H](C2CCCCC2)n2c(nc3cc(F)c(F)cc23)-c2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF4N3O3/c29-17-8-6-15(7-9-17)26-34-22-12-18(30)19(31)13-23(22)36(26)25(14-4-2-1-3-5-14)27(37)35-24-20(32)10-16(28(38)39)11-21(24)33/h6-14,25H,1-5H2,(H,35,37)(H,38,39)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human FXR by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1134-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.123
BindingDB Entry DOI: 10.7270/Q2MS3T1N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM10947

((1R,10R)-16-methyl-14-{3-[methyl({10-[methyl({3-[(...)Show SMILES CN(CCCCCCCCCCN(C)CCC(=O)N1CCC[C@@H]2C3Cc4[nH]c(=O)ccc4[C@]12CC(C)=C3)CCC(=O)N1CCC[C@@H]2C3Cc4[nH]c(=O)ccc4[C@]12CC(C)=C3 |r,c:38,65,TLB:44:45:47.48.54:59.57.56,53:54:45:59.57.56,30:31:22:36.34.33,21:22:24.25.31:36.34.33,THB:35:34:22:24.25.31,49:48:45:59.57.56,26:25:22:36.34.33,58:57:45:47.48.54| Show InChI InChI=1S/C50H72N6O4/c1-35-29-37-31-43-41(17-19-45(57)51-43)49(33-35)39(37)15-13-25-55(49)47(59)21-27-53(3)23-11-9-7-5-6-8-10-12-24-54(4)28-22-48(60)56-26-14-16-40-38-30-36(2)34-50(40,56)42-18-20-46(58)52-44(42)32-38/h17-20,29-30,37-40H,5-16,21-28,31-34H2,1-4H3,(H,51,57)(H,52,58)/t37?,38?,39-,40-,49-,50-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.93 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Shanghai Institute of Materia Medica
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... |
J Med Chem 48: 655-7 (2005)
Article DOI: 10.1021/jm0496178
BindingDB Entry DOI: 10.7270/Q2FQ9TT0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386751

(CHEMBL2046884)Show SMILES O=C(Nc1nnc(Sc2ncnc3cc(OCCCN4CCOCC4)ccc23)s1)Nc1cccc(c1)C#C Show InChI InChI=1S/C26H25N7O3S2/c1-2-18-5-3-6-19(15-18)29-24(34)30-25-31-32-26(38-25)37-23-21-8-7-20(16-22(21)27-17-28-23)36-12-4-9-33-10-13-35-14-11-33/h1,3,5-8,15-17H,4,9-14H2,(H2,29,30,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386750

(CHEMBL2046699)Show InChI InChI=1S/C13H11N3O2S2/c1-17-10-5-8-9(6-11(10)18-2)15-7-16-12(8)20-13-14-3-4-19-13/h3-7H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50336378
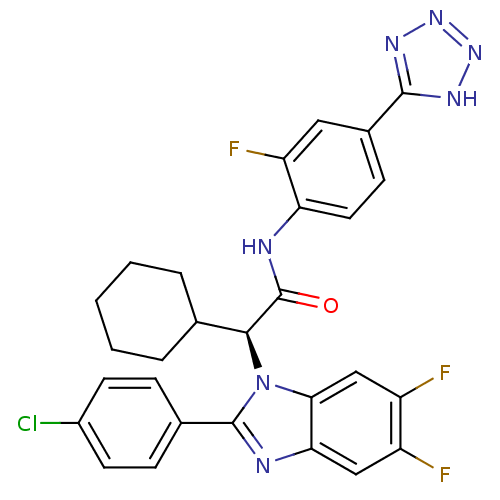
((S)-2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo[d]...)Show SMILES Fc1cc2nc(-c3ccc(Cl)cc3)n([C@@H](C3CCCCC3)C(=O)Nc3ccc(cc3F)-c3nnn[nH]3)c2cc1F |r| Show InChI InChI=1S/C28H23ClF3N7O/c29-18-9-6-16(7-10-18)27-33-23-13-19(30)20(31)14-24(23)39(27)25(15-4-2-1-3-5-15)28(40)34-22-11-8-17(12-21(22)32)26-35-37-38-36-26/h6-15,25H,1-5H2,(H,34,40)(H,35,36,37,38)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human FXR by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1134-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.123
BindingDB Entry DOI: 10.7270/Q2MS3T1N |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50336377
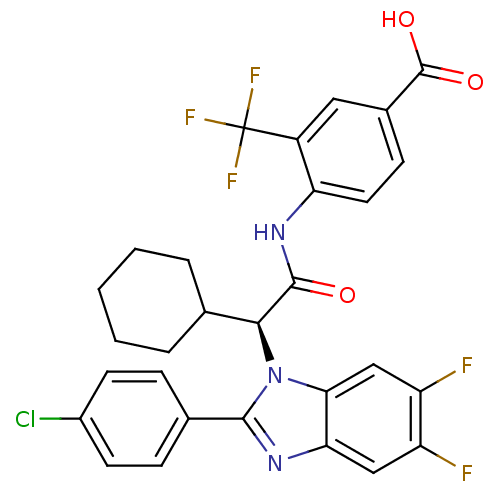
((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](C2CCCCC2)n2c(nc3cc(F)c(F)cc23)-c2ccc(Cl)cc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H23ClF5N3O3/c30-18-9-6-16(7-10-18)26-36-23-13-20(31)21(32)14-24(23)38(26)25(15-4-2-1-3-5-15)27(39)37-22-11-8-17(28(40)41)12-19(22)29(33,34)35/h6-15,25H,1-5H2,(H,37,39)(H,40,41)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human FXR by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1134-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.123
BindingDB Entry DOI: 10.7270/Q2MS3T1N |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386748
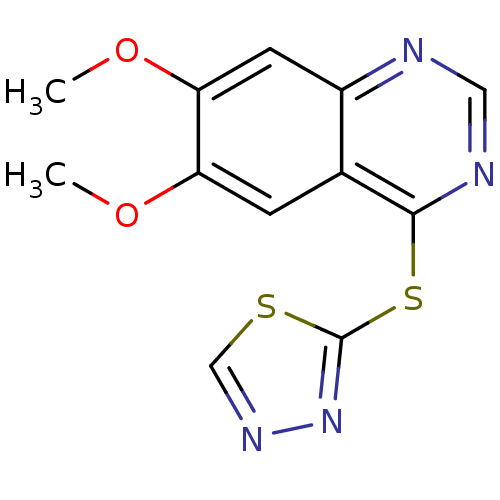
(CHEMBL2046726)Show InChI InChI=1S/C12H10N4O2S2/c1-17-9-3-7-8(4-10(9)18-2)13-5-14-11(7)20-12-16-15-6-19-12/h3-6H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data