 Found 824 hits with Last Name = 'liu' and Initial = 'jj'
Found 824 hits with Last Name = 'liu' and Initial = 'jj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449762
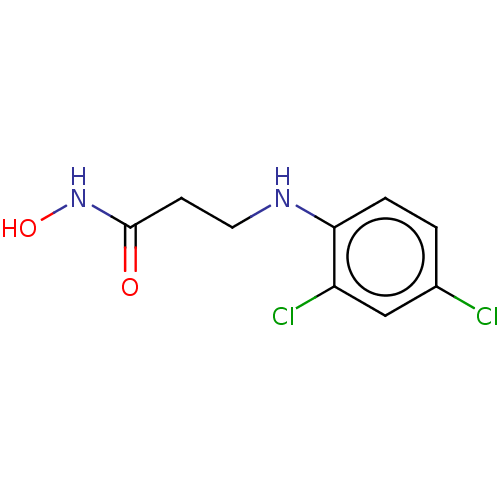
(CHEMBL4172924)Show InChI InChI=1S/C9H10Cl2N2O2/c10-6-1-2-8(7(11)5-6)12-4-3-9(14)13-15/h1-2,5,12,15H,3-4H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449754

(CHEMBL4167553)Show InChI InChI=1S/C9H10Cl2N2O2/c10-6-3-7(11)5-8(4-6)12-2-1-9(14)13-15/h3-5,12,15H,1-2H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449763
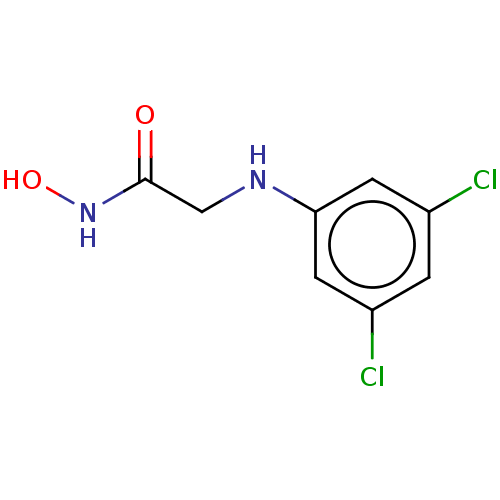
(CHEMBL4176964)Show InChI InChI=1S/C8H8Cl2N2O2/c9-5-1-6(10)3-7(2-5)11-4-8(13)12-14/h1-3,11,14H,4H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449762

(CHEMBL4172924)Show InChI InChI=1S/C9H10Cl2N2O2/c10-6-1-2-8(7(11)5-6)12-4-3-9(14)13-15/h1-2,5,12,15H,3-4H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449754

(CHEMBL4167553)Show InChI InChI=1S/C9H10Cl2N2O2/c10-6-3-7(11)5-8(4-6)12-2-1-9(14)13-15/h3-5,12,15H,1-2H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449763

(CHEMBL4176964)Show InChI InChI=1S/C8H8Cl2N2O2/c9-5-1-6(10)3-7(2-5)11-4-8(13)12-14/h1-3,11,14H,4H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... |
Eur J Med Chem 156: 126-136 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.065
BindingDB Entry DOI: 10.7270/Q2N87DBC |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053225
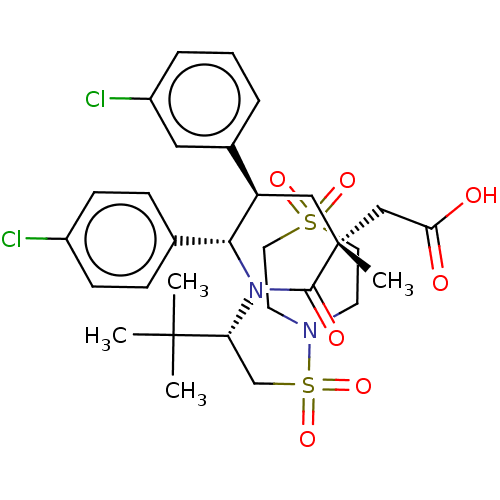
(CHEMBL3318783)Show SMILES CC(C)(C)[C@@H](CS(=O)(=O)N1CCS(=O)(=O)CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H38Cl2N2O7S2/c1-29(2,3)25(19-43(40,41)33-12-14-42(38,39)15-13-33)34-27(20-8-10-22(31)11-9-20)24(21-6-5-7-23(32)16-21)17-30(4,28(34)37)18-26(35)36/h5-11,16,24-25,27H,12-15,17-19H2,1-4H3,(H,35,36)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053046
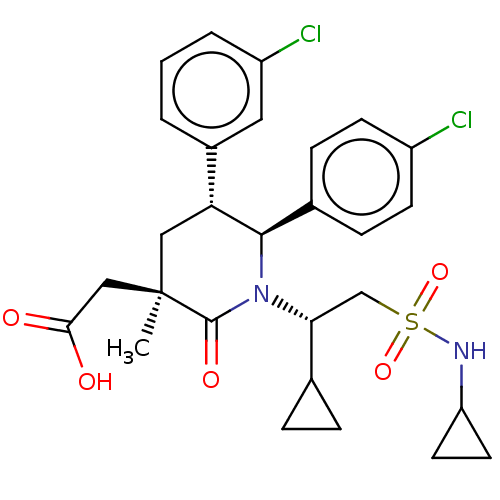
(CHEMBL3318761)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NC2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H32Cl2N2O5S/c1-28(15-25(33)34)14-23(19-3-2-4-21(30)13-19)26(18-7-9-20(29)10-8-18)32(27(28)35)24(17-5-6-17)16-38(36,37)31-22-11-12-22/h2-4,7-10,13,17,22-24,26,31H,5-6,11-12,14-16H2,1H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053053
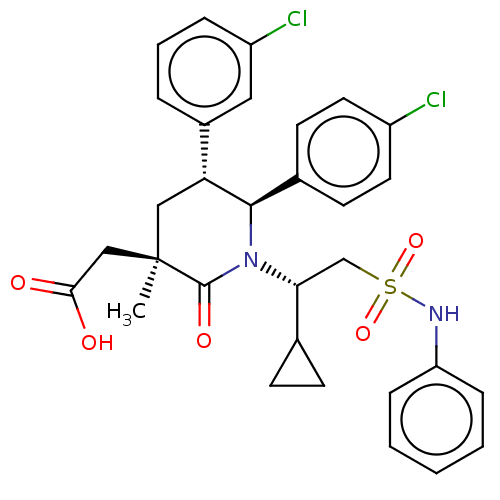
(CHEMBL3318768)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)Nc2ccccc2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C31H32Cl2N2O5S/c1-31(18-28(36)37)17-26(22-6-5-7-24(33)16-22)29(21-12-14-23(32)15-13-21)35(30(31)38)27(20-10-11-20)19-41(39,40)34-25-8-3-2-4-9-25/h2-9,12-16,20,26-27,29,34H,10-11,17-19H2,1H3,(H,36,37)/t26-,27-,29-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053223
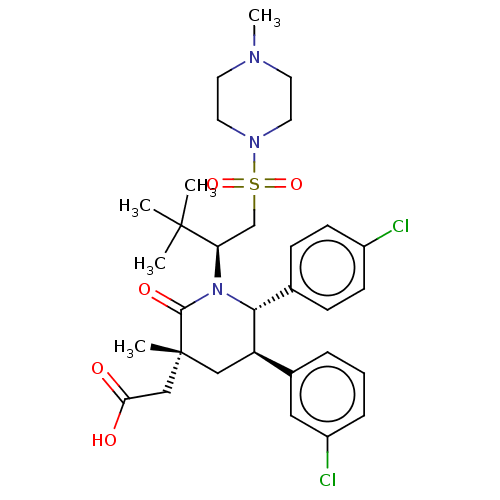
(CHEMBL3318781)Show SMILES CN1CCN(CC1)S(=O)(=O)C[C@@H](N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)C(C)(C)C |r| Show InChI InChI=1S/C31H41Cl2N3O5S/c1-30(2,3)26(20-42(40,41)35-15-13-34(5)14-16-35)36-28(21-9-11-23(32)12-10-21)25(22-7-6-8-24(33)17-22)18-31(4,29(36)39)19-27(37)38/h6-12,17,25-26,28H,13-16,18-20H2,1-5H3,(H,37,38)/t25-,26-,28-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053056
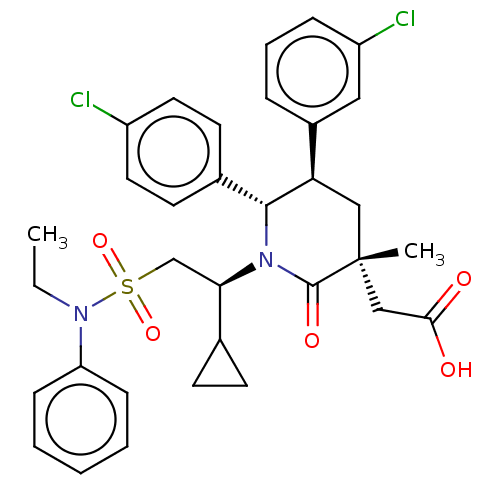
(CHEMBL3318771)Show SMILES CCN(c1ccccc1)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C33H36Cl2N2O5S/c1-3-36(27-10-5-4-6-11-27)43(41,42)21-29(22-12-13-22)37-31(23-14-16-25(34)17-15-23)28(24-8-7-9-26(35)18-24)19-33(2,32(37)40)20-30(38)39/h4-11,14-18,22,28-29,31H,3,12-13,19-21H2,1-2H3,(H,38,39)/t28-,29-,31-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053044
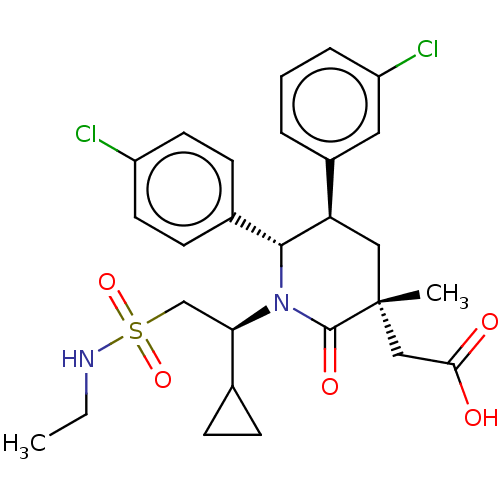
(CHEMBL3318756)Show SMILES CCNS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H32Cl2N2O5S/c1-3-30-37(35,36)16-23(17-7-8-17)31-25(18-9-11-20(28)12-10-18)22(19-5-4-6-21(29)13-19)14-27(2,26(31)34)15-24(32)33/h4-6,9-13,17,22-23,25,30H,3,7-8,14-16H2,1-2H3,(H,32,33)/t22-,23-,25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053042
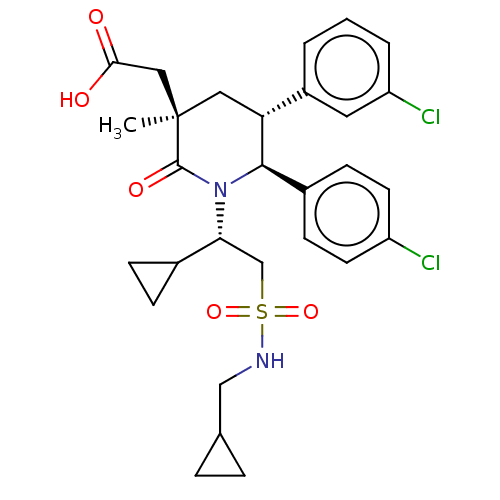
(CHEMBL3318758)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NCC2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(15-26(34)35)14-24(21-3-2-4-23(31)13-21)27(20-9-11-22(30)12-10-20)33(28(29)36)25(19-7-8-19)17-39(37,38)32-16-18-5-6-18/h2-4,9-13,18-19,24-25,27,32H,5-8,14-17H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
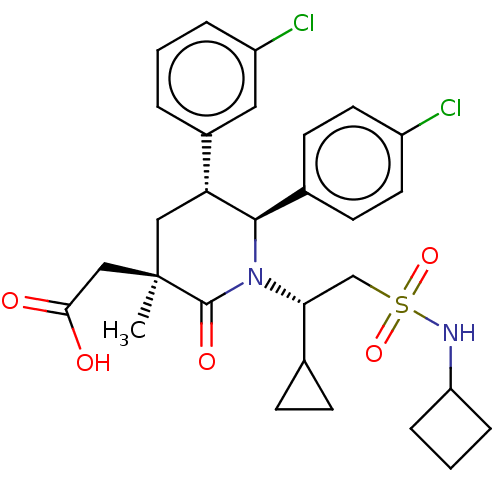
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053047
(CHEMBL3318762)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NC2CCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(16-26(34)35)15-24(20-4-2-5-22(31)14-20)27(19-10-12-21(30)13-11-19)33(28(29)36)25(18-8-9-18)17-39(37,38)32-23-6-3-7-23/h2,4-5,10-14,18,23-25,27,32H,3,6-9,15-17H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
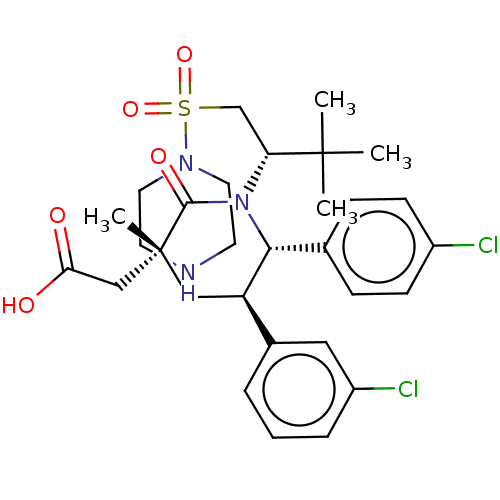
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053224
(CHEMBL3318782)Show SMILES CC(C)(C)[C@@H](CS(=O)(=O)N1CCNCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H39Cl2N3O5S/c1-29(2,3)25(19-41(39,40)34-14-12-33-13-15-34)35-27(20-8-10-22(31)11-9-20)24(21-6-5-7-23(32)16-21)17-30(4,28(35)38)18-26(36)37/h5-11,16,24-25,27,33H,12-15,17-19H2,1-4H3,(H,36,37)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
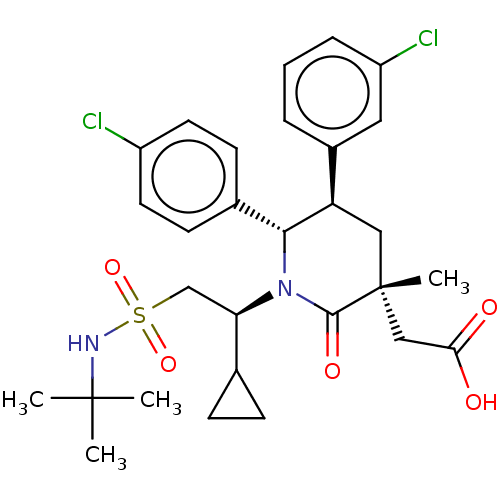
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053049
(CHEMBL3318764)Show SMILES CC(C)(C)NS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H36Cl2N2O5S/c1-28(2,3)32-39(37,38)17-24(18-8-9-18)33-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(33)36)16-25(34)35/h5-7,10-14,18,23-24,26,32H,8-9,15-17H2,1-4H3,(H,34,35)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053052
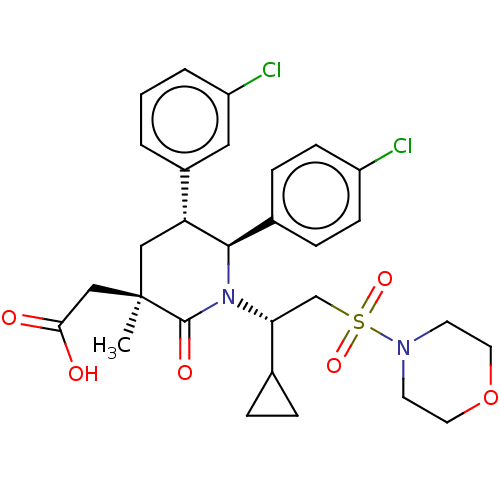
(CHEMBL3318767 | US9296736, 379 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCOCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O6S/c1-29(17-26(34)35)16-24(21-3-2-4-23(31)15-21)27(20-7-9-22(30)10-8-20)33(28(29)36)25(19-5-6-19)18-40(37,38)32-11-13-39-14-12-32/h2-4,7-10,15,19,24-25,27H,5-6,11-14,16-18H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data