

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Rattus norvegicus) | BDBM50136199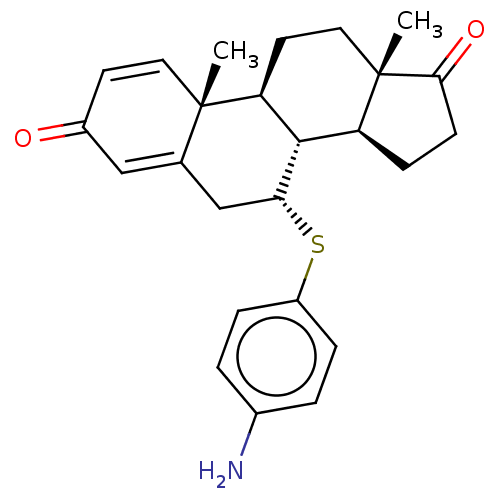 (CHEMBL3349548) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114658 BindingDB Entry DOI: 10.7270/Q2NP28CD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014320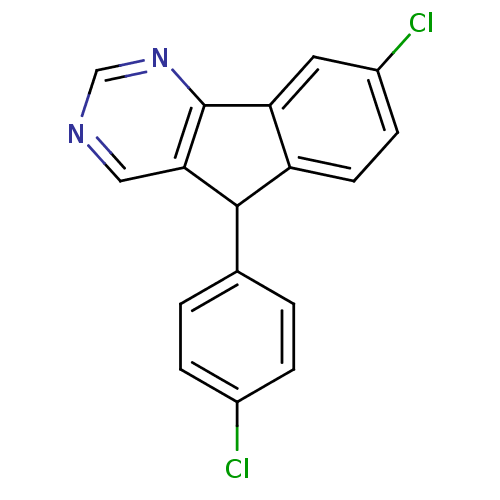 (8-Chloro-5-(4-chloro-phenyl)-5H-indeno[1,2-d]pyrim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014324 (5-[Bis-(4-chloro-phenyl)-methyl]-pyrimidine | 5-[B...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014315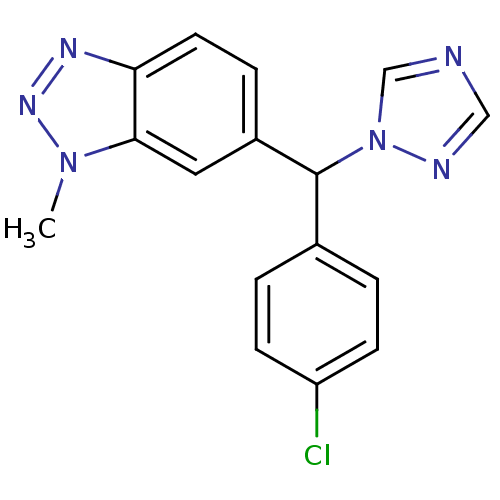 ((rac)-6-((4-chlorophenyl)(1H-1,2,4-triazol-1-yl)me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50049763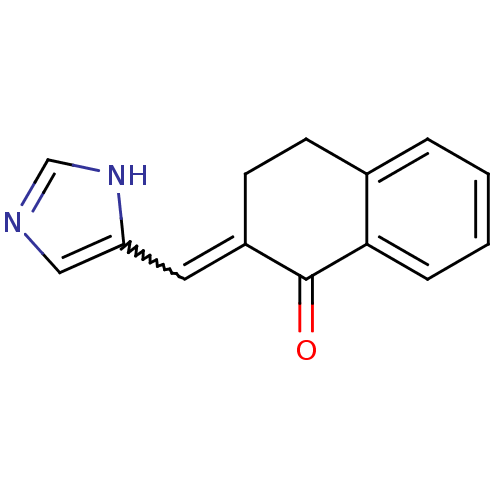 (2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah Curated by ChEMBL | Assay Description Inhibition of rat ovarian aromatase | Eur J Med Chem 102: 375-86 (2015) Article DOI: 10.1016/j.ejmech.2015.08.010 BindingDB Entry DOI: 10.7270/Q2ZC84Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50123022 (CHEMBL169251) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah Curated by ChEMBL | Assay Description Inhibition of rat ovarian aromatase | Eur J Med Chem 102: 375-86 (2015) Article DOI: 10.1016/j.ejmech.2015.08.010 BindingDB Entry DOI: 10.7270/Q2ZC84Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian microsomal Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50009606 (5-Hydroxy-2-pyridin-4-ylmethyl-3,4-dihydro-2H-naph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian aromatase cytochrome P450 19A1 | J Med Chem 34: 2685-91 (1991) BindingDB Entry DOI: 10.7270/Q2ST7NT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50009605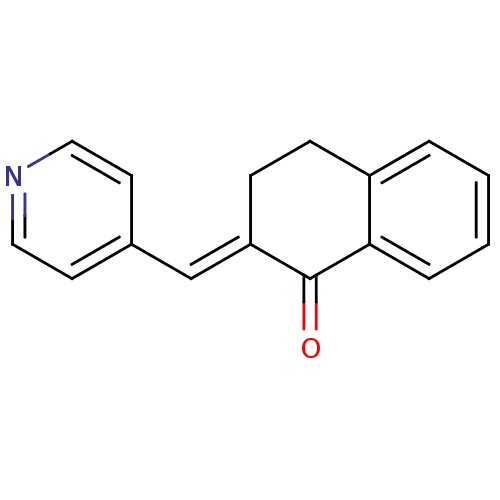 (2-(pyridin-4-ylmethylene)-3,4-dihydronaphthalen-1(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah Curated by ChEMBL | Assay Description Inhibition of rat ovarian aromatase | Eur J Med Chem 102: 375-86 (2015) Article DOI: 10.1016/j.ejmech.2015.08.010 BindingDB Entry DOI: 10.7270/Q2ZC84Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian microsomal Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50009618 (5-Methoxy-2-pyridin-4-ylmethyl-3,4-dihydro-2H-naph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian aromatase cytochrome P450 19A1 | J Med Chem 34: 2685-91 (1991) BindingDB Entry DOI: 10.7270/Q2ST7NT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of rat ovarian Cytochrome P450 19A | J Med Chem 34: 2685-91 (1991) BindingDB Entry DOI: 10.7270/Q2ST7NT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024479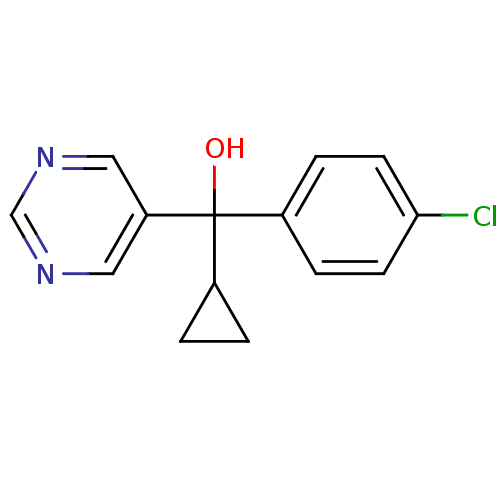 ((4-Chloro-phenyl)-cyclopropyl-pyrimidin-5-yl-metha...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024480 ((3,4-Dichloro-phenyl)-phenyl-pyrimidin-5-yl-methan...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024481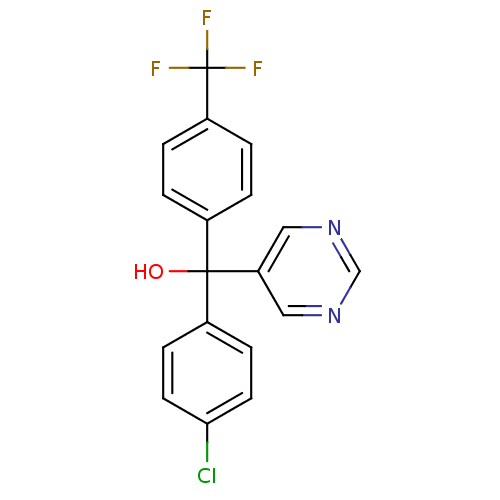 ((4-Chloro-phenyl)-pyrimidin-5-yl-(4-trifluoromethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024482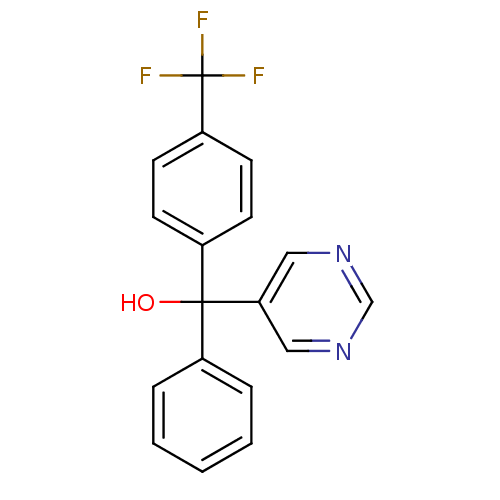 (CHEMBL284636 | Phenyl-pyrimidin-5-yl-(4-trifluorom...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024483 ((4-Nitro-phenyl)-phenyl-pyrimidin-5-yl-methanol | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024484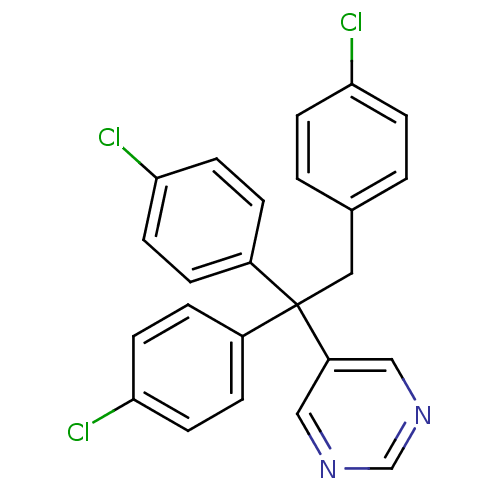 (5-[1,1,2-Tris-(4-chloro-phenyl)-ethyl]-pyrimidine ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024485 (5-[1,1-Bis-(4-chloro-phenyl)-2-phenyl-ethyl]-pyrim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024486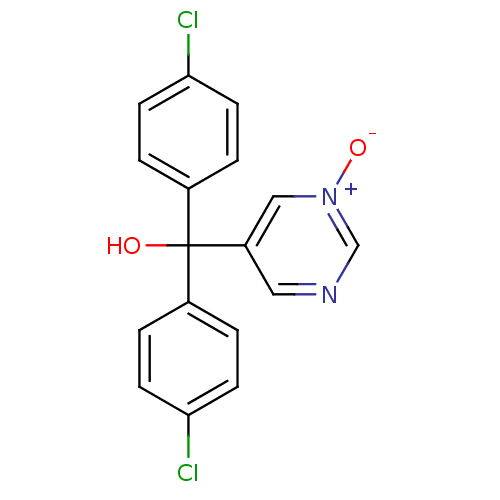 (Bis-(4-chloro-phenyl)-(1-oxy-pyrimidin-5-yl)-metha...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024487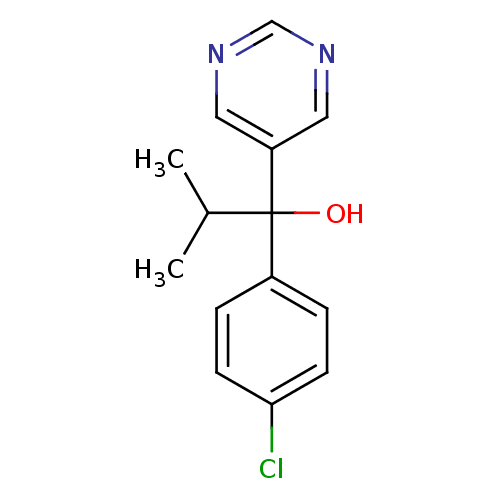 (1-(4-Chloro-phenyl)-2-methyl-1-pyrimidin-5-yl-prop...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024489 (5-[Bis-(4-chloro-phenyl)-methylene]-1,4,5,6-tetrah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024490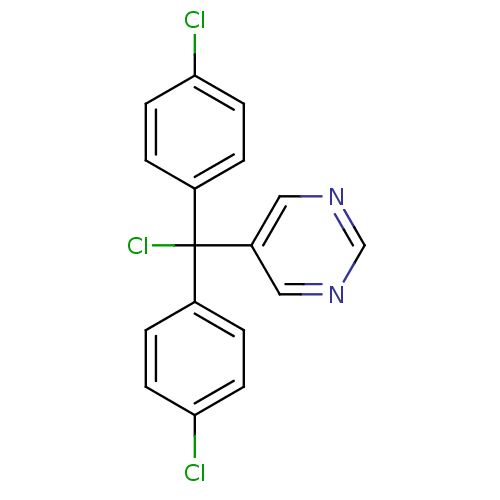 (5-[Chloro-bis-(4-chloro-phenyl)-methyl]-pyrimidine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 275 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024488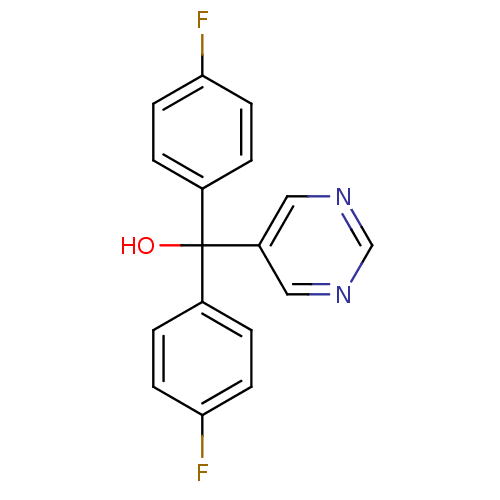 (Bis-(4-fluoro-phenyl)-pyrimidin-5-yl-methanol | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM31772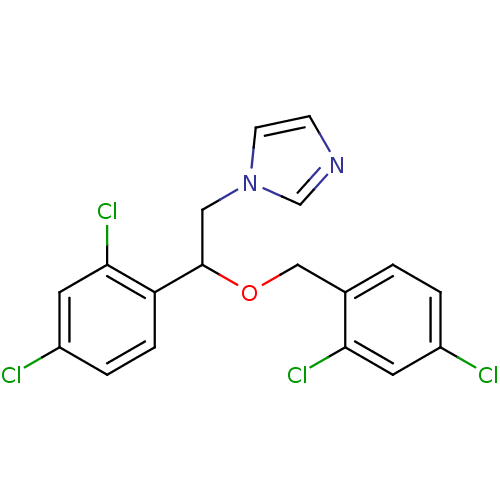 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024491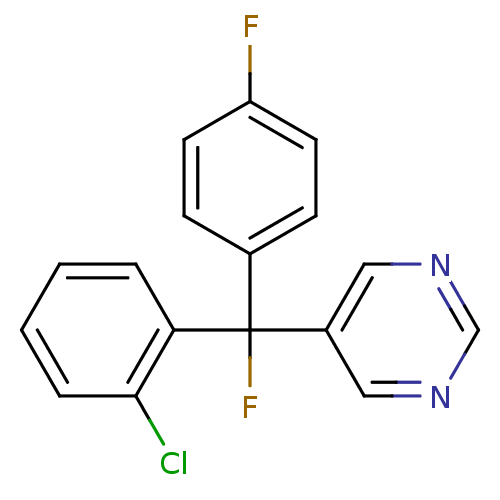 (5-[(2-Chloro-phenyl)-fluoro-(4-fluoro-phenyl)-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024492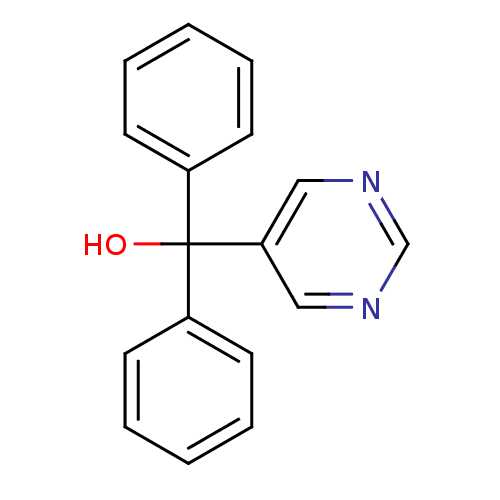 (CHEMBL29670 | Diphenyl-pyrimidin-5-yl-methanol) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014324 (5-[Bis-(4-chloro-phenyl)-methyl]-pyrimidine | 5-[B...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024494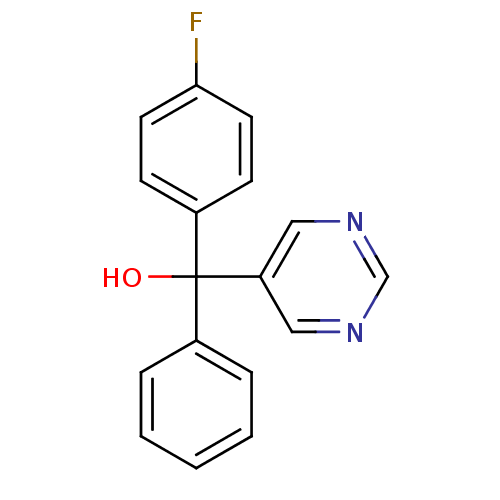 ((4-Fluoro-phenyl)-phenyl-pyrimidin-5-yl-methanol |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >2.20E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024493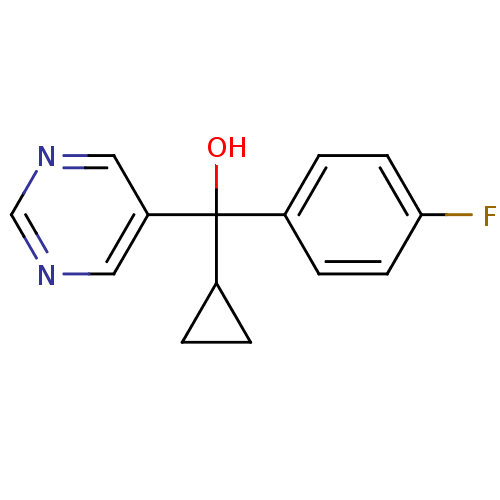 (CHEMBL30081 | Cyclopropyl-(4-fluoro-phenyl)-pyrimi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024495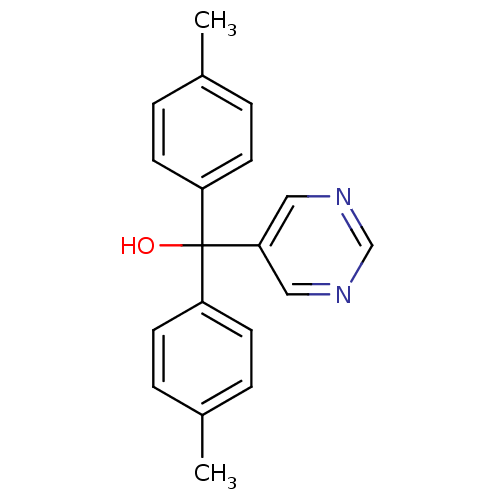 (CHEMBL283085 | Pyrimidin-5-yl-di-p-tolyl-methanol) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024497 (2-Methyl-1-(5-methyl-thiophen-2-yl)-1-pyrimidin-5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024496 (CHEMBL29412 | Pyrimidin-5-yl-di-thiophen-2-yl-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024498 (5-[1,1-Bis-(4-chloro-phenyl)-propyl]-pyrimidine | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024499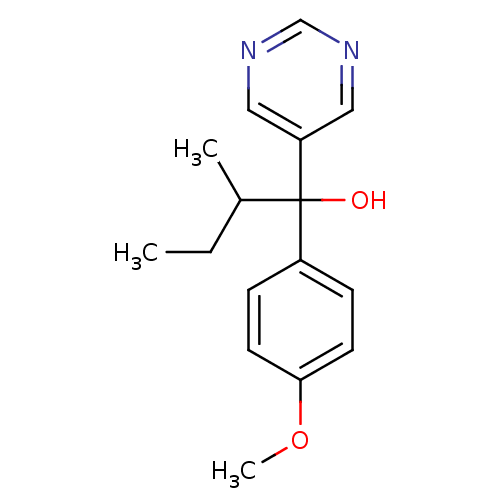 (1-(4-Methoxy-phenyl)-2-methyl-1-pyrimidin-5-yl-but...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024500 (CHEMBL29140 | Phenyl-pyrimidin-5-yl-thiophen-2-yl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024501 ((4-Bromo-phenyl)-cyclopropyl-pyrimidin-5-yl-methan...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024502 (1-(4-Methoxy-phenyl)-2,2-dimethyl-1-pyrimidin-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024503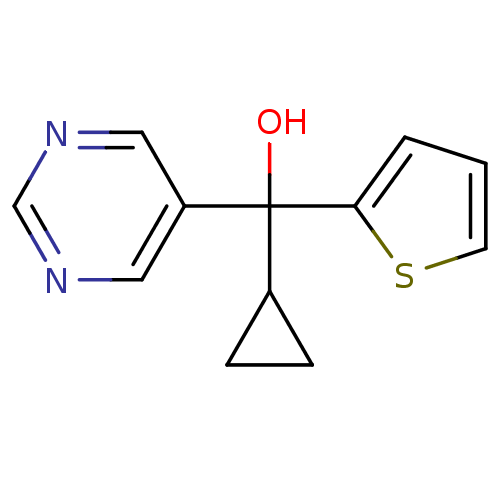 (CHEMBL28860 | Cyclopropyl-pyrimidin-5-yl-thiophen-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024504 ((2-Chloro-4-methoxy-phenyl)-(4-chloro-phenyl)-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024506 ((4-Chloro-phenyl)-phenyl-pyrimidin-5-yl-methanol |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024505 ((3,4-Dimethyl-phenyl)-(4-methoxy-3-methyl-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024507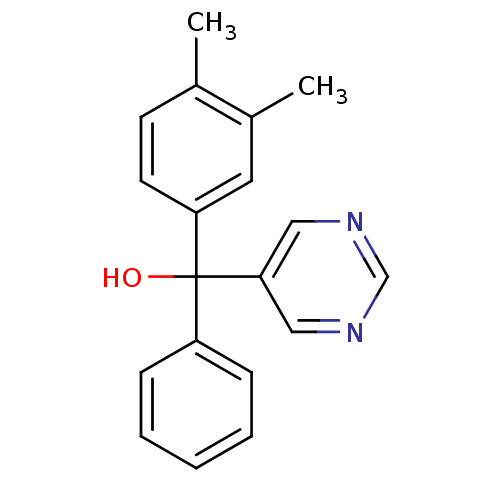 ((3,4-Dimethyl-phenyl)-phenyl-pyrimidin-5-yl-methan...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024512 (CHEMBL280781 | Cyclohexyl-pyrimidin-5-yl-p-tolyl-m...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024511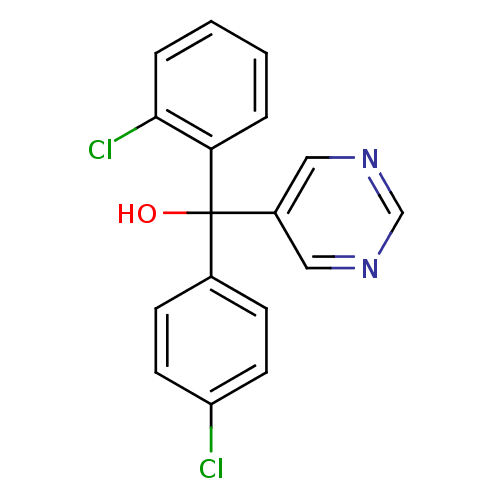 ((4-Chloro-phenyl)-(2-chloro-phenyl)-pyrimidin-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024508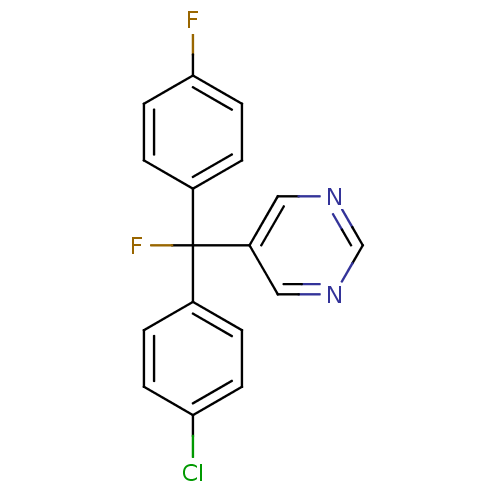 (5-[(4-Chloro-phenyl)-fluoro-(4-fluoro-phenyl)-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024510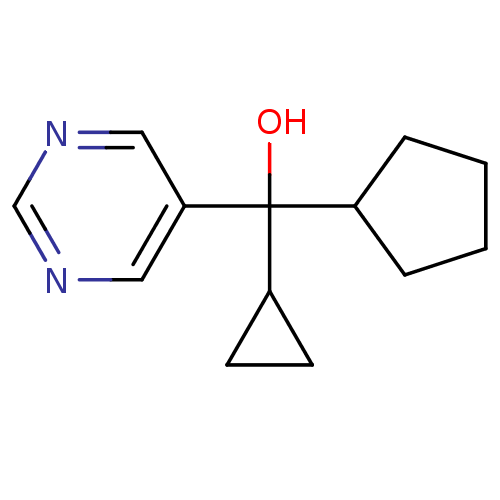 (CHEMBL417120 | Cyclopentyl-cyclopropyl-pyrimidin-5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024509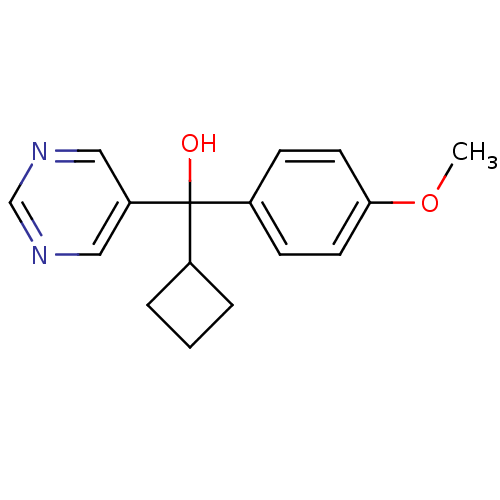 (CHEMBL285844 | Cyclobutyl-(4-methoxy-phenyl)-pyrim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024513 (6-Acetylsulfanyl-8-[2-[(4-amino-2-methyl-pyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131 total ) | Next | Last >> |