

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50252861 CHEMBL507214::H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]-Thr-NH2
SMILES: C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O
InChI Key: InChIKey=PZWWYAHWHHNCHO-FGHAYEPSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50252861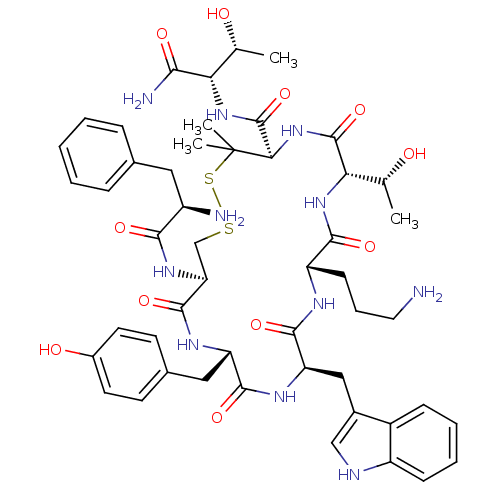 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Antagonist activity against delta opioid receptor in mouse vas deference assessed as effect on DPDPE-induced response | J Med Chem 51: 5866-70 (2008) Article DOI: 10.1021/jm8004702 BindingDB Entry DOI: 10.7270/Q20G3K0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for maximum agonist response at melanocortin 1 receptor from frog skin. | J Med Chem 47: 1514-26 (2004) Article DOI: 10.1021/jm030452x BindingDB Entry DOI: 10.7270/Q2XP75PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE binding to Opioid receptor delta 1 of rat brain membranes | J Med Chem 29: 2370-5 (1986) BindingDB Entry DOI: 10.7270/Q2SQ910T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]naloxone toOpioid receptor mu 1 of rat brain membranes | J Med Chem 29: 2370-5 (1986) BindingDB Entry DOI: 10.7270/Q2SQ910T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Antagonist activity against mu opioid receptor in guinea pig ileum assessed as effect on TAPP-induced response | J Med Chem 51: 5866-70 (2008) Article DOI: 10.1021/jm8004702 BindingDB Entry DOI: 10.7270/Q20G3K0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||