 Found 18 hits for monomerid = 50101824
Found 18 hits for monomerid = 50101824 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101824
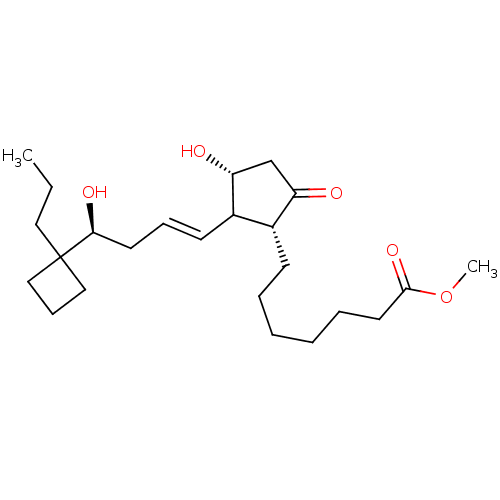
(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards human Prostanoid IP receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP1 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 2A1
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP2 receptor expressed in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(BOVINE) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Laboratories Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1094-102 (1998)
BindingDB Entry DOI: 10.7270/Q2KK99BC |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP4 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration which increases intracellular c-AMP production in mouse EP2- receptor |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101824

(7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19?,21-,22+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration which increases intracellular c-AMP production in human Prostanoid IP receptor |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data