

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50161511 1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-one::1-(5-(furan-2-yl)-1,3,4-oxadiazol-2-yl)-7-phenylheptan-1-one::1-[5-(furan-2-yl)-1,3,4-oxadiazol-2-yl]-7-phenylheptan-1-one::CHEMBL426967
SMILES: O=C(CCCCCCc1ccccc1)c1nnc(o1)-c1ccco1
InChI Key: InChIKey=FKFVIVTXKDACMG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM50161511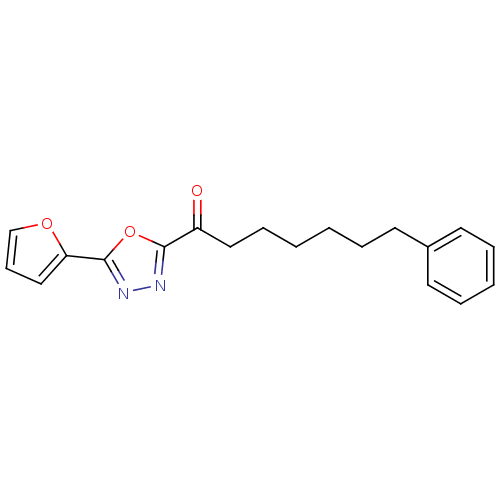 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of rat FAAH | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anandamide amidohydrolase (Mus musculus (mouse)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in Escherichia coli at pH 9.0 | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sn1-specific diacylglycerol lipase alpha (Homo sapiens (Human)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of full-length human DAGLalpha expressed in HEK293T cell membranes using para-nitrophenylbutyrate by colorimetric assay | J Med Chem 58: 9742-53 (2015) Article DOI: 10.1021/acs.jmedchem.5b01627 BindingDB Entry DOI: 10.7270/Q2D50QZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in Escherichia coli | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral cholesterol ester hydrolase 1 (Homo sapiens (Human)) | BDBM50161511 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of KIAA1363 hydrolase | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||