

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50211215 CHEMBL3898922
SMILES: C1N=C(Nc2ccccn2)O[C@]11CN2CCC1CC2
InChI Key: InChIKey=NWOGQPOGFKBIMN-AWEZNQCLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211215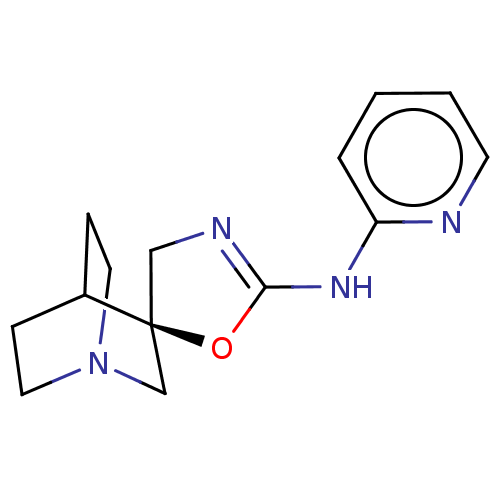 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50211215 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells co-expressing human RIC3 measured for 2 mins by FLIPR assay | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50211215 (CHEMBL3898922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at alpha4beta2 nAChR (unknown origin) assessed as increase in calcium influx measured for 2 mins by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211215 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT3A receptor assessed as inhibition of 5-HT-induced calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha3/beta4 (Homo sapiens (Human)) | BDBM50211215 (CHEMBL3898922) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at alpha3beta4 nAChR (unknown origin) assessed as increase in calcium influx measured for 2 mins by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50211215 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as increase in calcium influx measured for 2 mins by Fluo-4-AM dye based FLIP... | Bioorg Med Chem Lett 27: 1261-1266 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor alpha-1/beta-1/delta/epsilon (Homo sapiens (Human)) | BDBM50211215 (CHEMBL3898922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at alpha1beta1deltaepsilon nAChR (unknown origin) assessed as increase in calcium influx measured for 2 mins by Fluo-4-AM dye based ... | Bioorg Med Chem Lett 27: 1261-1266 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||