

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50286739 4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one::4-((1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one::4-[(1R,3S,5S,10R,11R,13R,14S,17R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one::4-[(R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one::4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one::4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one(Ouabain)::CHEMBL222863::NSC-25485::Ouabain::Ouabain4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one::cid_439501
SMILES: C[C@@H]1O[C@@H](O[C@H]2C[C@@H](O)[C@]3(CO)[C@H]4[C@H](O)C[C@]5(C)[C@H](CC[C@]5(O)[C@@H]4CC[C@]3(O)C2)C2=CC(=O)OC2)[C@H](O)[C@H](O)[C@H]1O
InChI Key: InChIKey=LPMXVESGRSUGHW-HBYQJFLCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na,K-ATPase alpha2beta1 (Homo sapiens (Human)) | BDBM50286739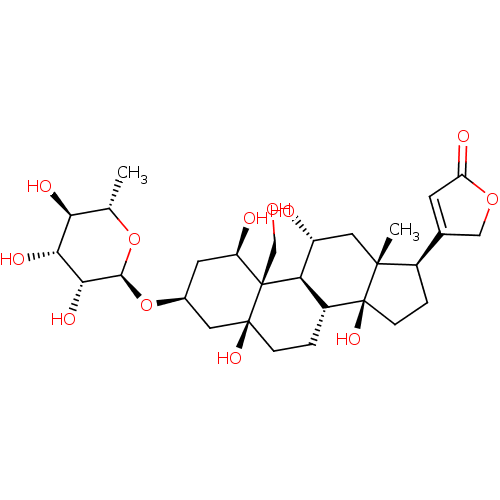 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weizmann Institute of Science | Assay Description Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d... | J Biol Chem 289: 21153-62 (2014) Article DOI: 10.1074/jbc.M114.557629 BindingDB Entry DOI: 10.7270/Q2G44P6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na,K-ATPase alpha1beta1 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weizmann Institute of Science | Assay Description Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d... | J Biol Chem 289: 21153-62 (2014) Article DOI: 10.1074/jbc.M114.557629 BindingDB Entry DOI: 10.7270/Q2G44P6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kruppel-like factor 5 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 29.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2251GNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat kidney Na+/K+ ATPase was determined by fiske subbarow method | Bioorg Med Chem Lett 5: 827-830 (1995) Article DOI: 10.1016/0960-894X(95)00121-9 BindingDB Entry DOI: 10.7270/Q2XK8FJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... | Drug Metab Dispos 40: 2332-41 (2012) Article DOI: 10.1124/dmd.112.047068 BindingDB Entry DOI: 10.7270/Q2ZP488M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na+/K+ ATPase alpha-3/beta-1 (Rattus norvegicus) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha3/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na+/K+ ATPase alpha-2/beta-1 (Rattus norvegicus) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha2/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na+/K+ ATPase alpha-1/beta-1 (RAT-Rattus norvegicus) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha1/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N+/K+ ATPase alpha-4/beta-1 (Rattus norvegicus) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N+/K+ ATPase alpha-4/beta-3 (Rattus norvegicus) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta3 expressed in baculovirus infected Sf9 cell membranes using [gamma-32P]ATP as substrate prein... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Displacement of Tracer Red from human ERG expressed in cell membranes by fluorescence polarization assay | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <97 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea | Assay Description Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ... | Antimicrob Agents Chemother 64: (2020) Article DOI: 10.1128/AAC.00819-20 BindingDB Entry DOI: 10.7270/Q22N54QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Inhibition of HIV1 RT | J Nat Prod 54: 143-54 Article DOI: 10.1021/np50073a012 BindingDB Entry DOI: 10.7270/Q2NK3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STAT3 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2TQ5ZXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription Factor STAT1 (Homo sapiens (Human)) | BDBM50286739 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | >5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K935ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||