

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50408438 (CHEMBL2068911) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50408439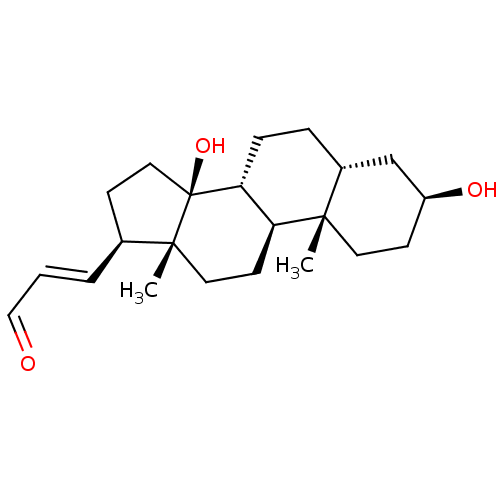 (CHEMBL2068890) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM66977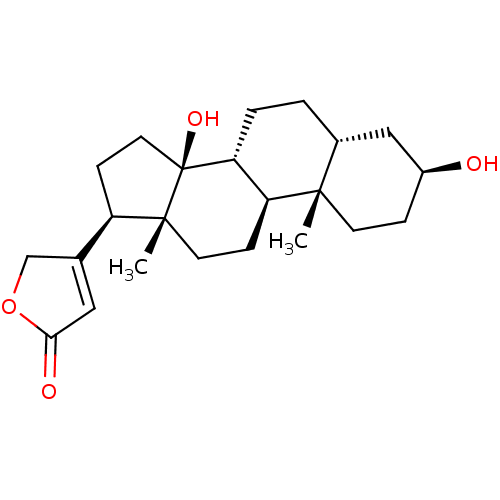 (3-[(3S,5R,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50066032 (CHEMBL323301 | [(1R,4bR,7S,8aR)-7-Hydroxy-1,4b,8,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50066031 ((E)-(S)-4-((2S,4bS,7S,8aR)-7-Hydroxy-2,4b-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50066029 (4-[(S)-1-((2S,4bS,7S,8aR)-7-Hydroxy-2,4b-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50066036 (4-[(R)-1-((2R,4bS,7S,8aR)-7-Hydroxy-2,4b-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50131828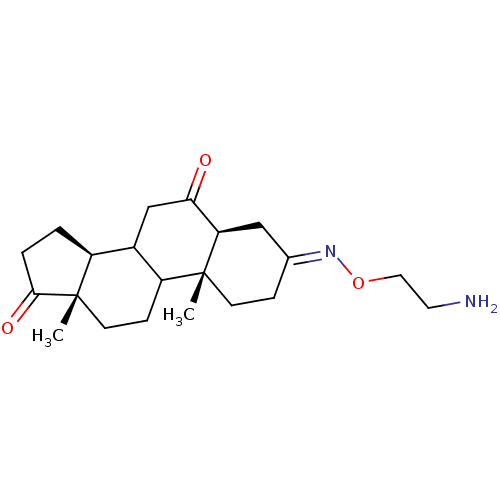 ((E,Z)10,13-Dimethyl-tetradecahydro-cyclopenta[a]ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Inhibitory activity against Na+/K+ ATPase | J Med Chem 46: 3644-54 (2003) Article DOI: 10.1021/jm030830y BindingDB Entry DOI: 10.7270/Q24T6K47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50066034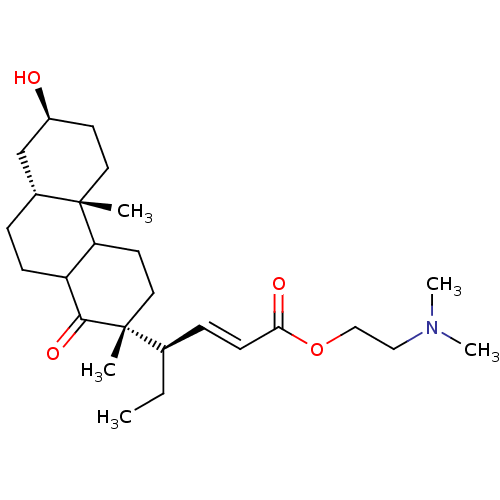 ((E)-(R)-4-((2R,4bS,7S,8aR)-7-Hydroxy-2,4b-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50408437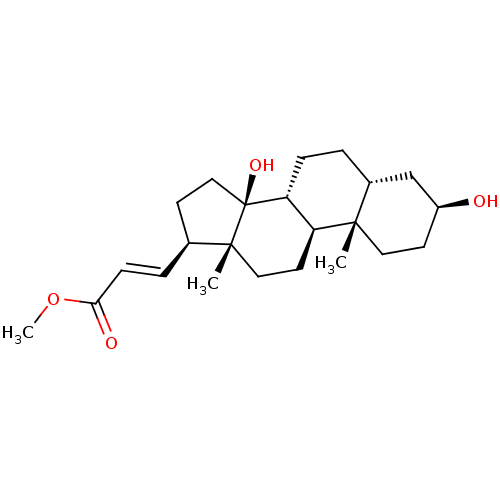 (CHEMBL2068917) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50408440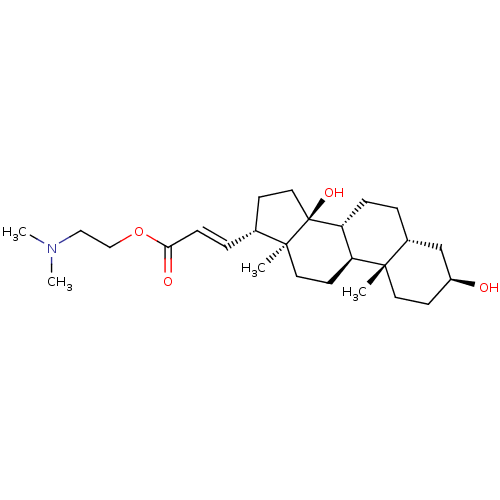 (CHEMBL2068987) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase | J Med Chem 41: 3033-40 (1998) Article DOI: 10.1021/jm980108d BindingDB Entry DOI: 10.7270/Q2VX0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50131829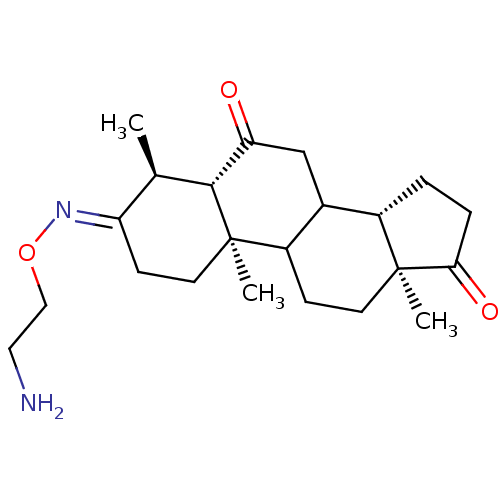 ((E)4,10,13-Trimethyl-tetradecahydro-cyclopenta[a]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Inhibitory activity against Na+/K+ ATPase | J Med Chem 46: 3644-54 (2003) Article DOI: 10.1021/jm030830y BindingDB Entry DOI: 10.7270/Q24T6K47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50131821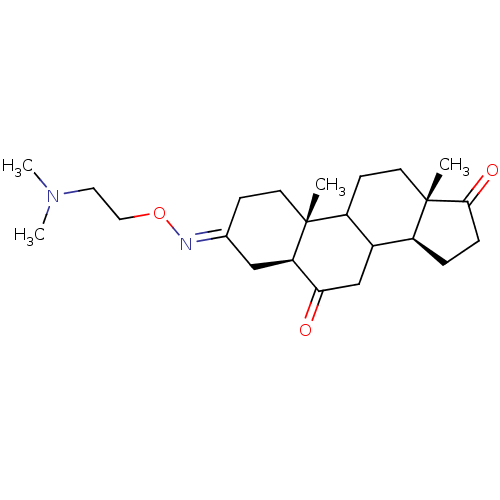 ((E,Z)10,13-Dimethyl-tetradecahydro-cyclopenta[a]ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Inhibitory activity against Na+/K+ ATPase measured by 32P-ATP hydrolysis method | J Med Chem 46: 3644-54 (2003) Article DOI: 10.1021/jm030830y BindingDB Entry DOI: 10.7270/Q24T6K47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50286741 (CHEMBL168713 | methyl 18-[6-(2-{2-[4,5-dihydroxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat kidney Na+/K+ ATPase was determined by fiske subbarow method | Bioorg Med Chem Lett 5: 827-830 (1995) Article DOI: 10.1016/0960-894X(95)00121-9 BindingDB Entry DOI: 10.7270/Q2XK8FJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50131832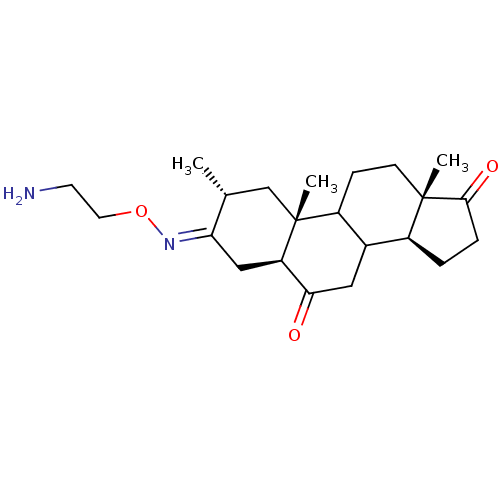 ((E)2,10,13-Trimethyl-tetradecahydro-cyclopenta[a]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Inhibitory activity against Na+/K+ ATPase | J Med Chem 46: 3644-54 (2003) Article DOI: 10.1021/jm030830y BindingDB Entry DOI: 10.7270/Q24T6K47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50013297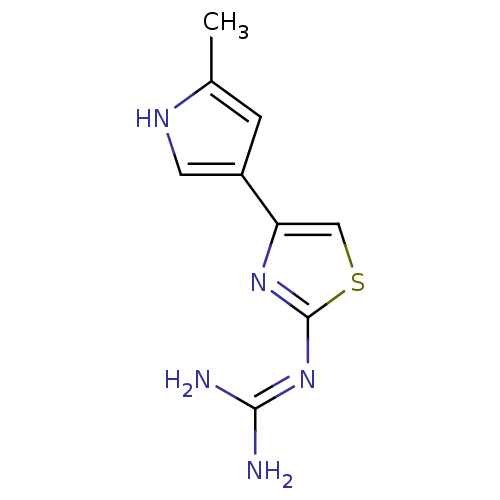 (CHEMBL149689 | N-[4-(5-Methyl-1H-pyrrol-3-yl)-thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Tested against canine renal Na+/K+ ATPase | J Med Chem 33: 543-52 (1990) BindingDB Entry DOI: 10.7270/Q2K35V79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50131823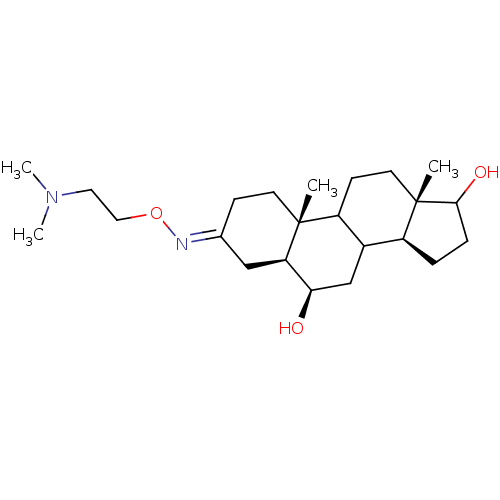 ((E,Z)6,17-Dihydroxy-10,13-dimethyl-hexadecahydro-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Prassis Istituto di Ricerche Sigma-Tau Curated by ChEMBL | Assay Description Inhibitory activity against Na+/K+ ATPase was determined in guinea pig | J Med Chem 46: 3644-54 (2003) Article DOI: 10.1021/jm030830y BindingDB Entry DOI: 10.7270/Q24T6K47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50286739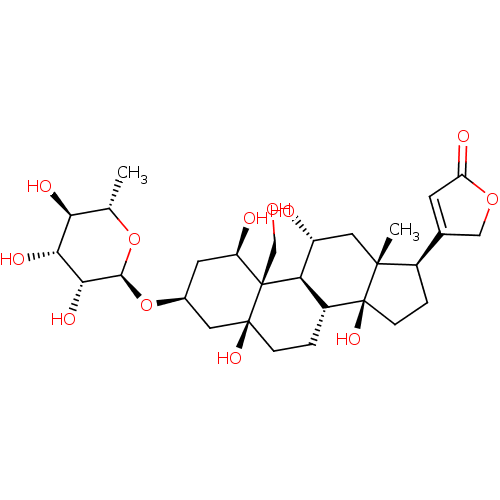 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat kidney Na+/K+ ATPase was determined by fiske subbarow method | Bioorg Med Chem Lett 5: 827-830 (1995) Article DOI: 10.1016/0960-894X(95)00121-9 BindingDB Entry DOI: 10.7270/Q2XK8FJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase alpha chain 2 (Homo sapiens (Human)) | BDBM50286740 (CHEMBL406262 | Saponin derivative) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat kidney Na+/K+ ATPase was determined by fiske subbarow method | Bioorg Med Chem Lett 5: 827-830 (1995) Article DOI: 10.1016/0960-894X(95)00121-9 BindingDB Entry DOI: 10.7270/Q2XK8FJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||