 Found 15 hits for monomerid = 50370682
Found 15 hits for monomerid = 50370682 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50370682
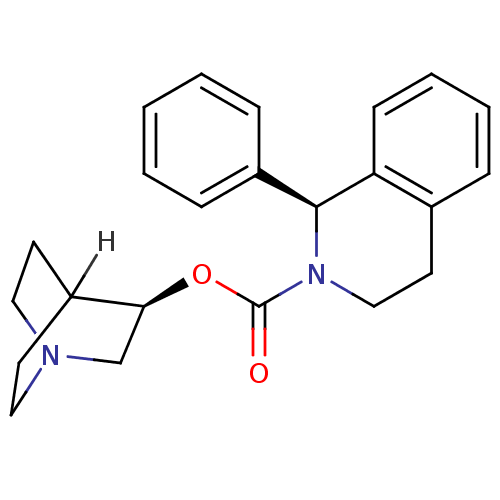
(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human muscarinic M3 receptor expressed in CHOK1 cell membranes after 120 mins by scintillation counting meth... |
Eur J Med Chem 137: 327-337 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.004
BindingDB Entry DOI: 10.7270/Q2VT1VMF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human muscarinic M5 receptor expressed in CHOK1 cell membranes after 120 mins by scintillation counting meth... |
Eur J Med Chem 137: 327-337 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.004
BindingDB Entry DOI: 10.7270/Q2VT1VMF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human muscarinic M1 receptor expressed in CHOK1 cell membranes after 120 mins by scintillation counting meth... |
Eur J Med Chem 137: 327-337 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.004
BindingDB Entry DOI: 10.7270/Q2VT1VMF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2 and M5
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human muscarinic M2 receptor expressed in CHOK1 cell membranes after 120 mins by scintillation counting meth... |
Eur J Med Chem 137: 327-337 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.004
BindingDB Entry DOI: 10.7270/Q2VT1VMF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2 and M4
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human muscarinic M4 receptor expressed in CHOK1 cell membranes after 120 mins by scintillation counting meth... |
Eur J Med Chem 137: 327-337 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.004
BindingDB Entry DOI: 10.7270/Q2VT1VMF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of transient outward potassium current (Ito) current in Chinese Hamster Ovary (CHO) K1 cells expressing human Kv4.3 measured using IonWork... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of rapid delayed inward rectifying potassium current (IKr) in Chinese hamster ovary (CHO) K1 cells stably expressing hERG measured using I... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |
Calcium channel (Type L)
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of fast sodium current (INa) in Chinese Hamster Ovary (CHO) K1 cells transfected with human Nav1.5 measured using IonWorks Quattro automat... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of rapid delayed inward rectifying potassium current (IKr) in Chinese hamster ovary (CHO) K1 cells stably expressing hERG measured using I... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |
Voltage-gated L-type calcium channel
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChanTest Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 current measured using QPatch automatic path clamp system in CHO cells expressing Cav1.2, beta-2 and alpha-2/delta-1 subunits |
Sci Rep 3: (2013)
Article DOI: 10.1038/srep02100
BindingDB Entry DOI: 10.7270/Q2RB77K2 |
More data for this
Ligand-Target Pair | |
Voltage-gated potassium channel subunit Kv4.3
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of rapid delayed inward rectifying potassium current (IKr) in Chinese hamster ovary (CHO) K1 cells stably expressing hERG measured using I... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |
Voltage-gated potassium channel beta subunit Mink/subunit Kv7.1
(Homo sapiens (Human)) | BDBM50370682

(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of slow delayed inward rectifying potassium current (Iks) in Chinese Hamster Ovary (CHO) cells expressing hKvLQT1/hminK measured using Ion... |
J Pharmacol Toxicol Methods 70: 246-54 (2014)
Article DOI: 10.1016/j.vascn.2014.07.002
BindingDB Entry DOI: 10.7270/Q2J104W7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data