

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163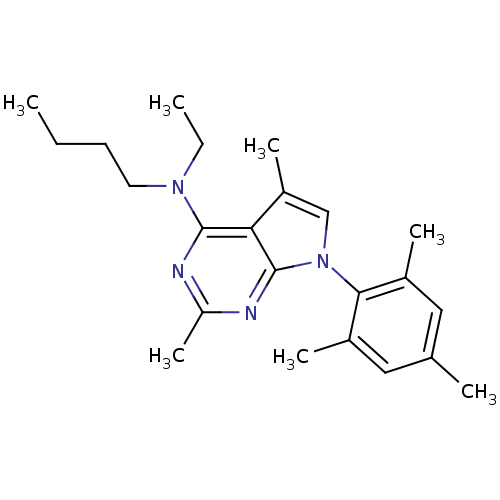 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135349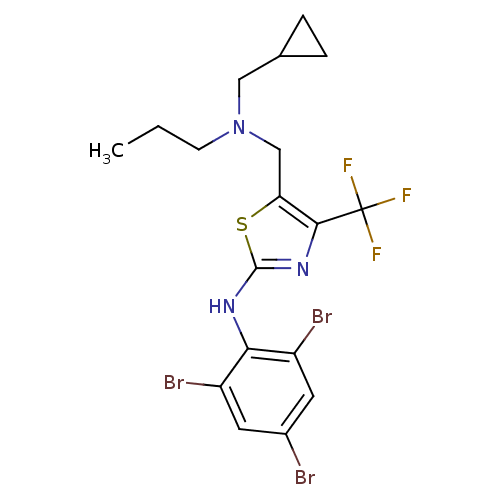 (CHEMBL341313 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135329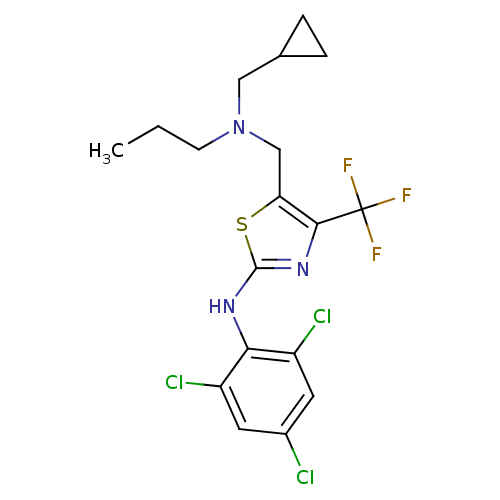 (CHEMBL128100 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135339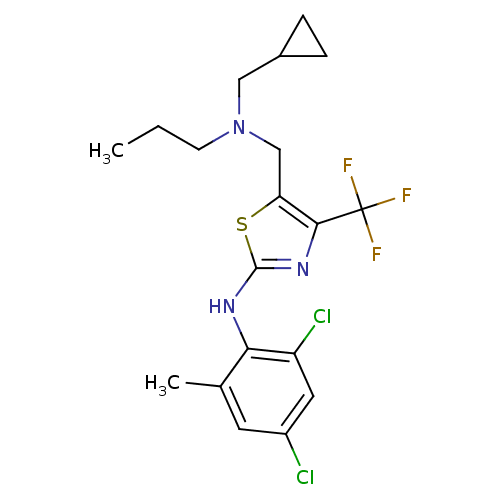 (CHEMBL128709 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50054245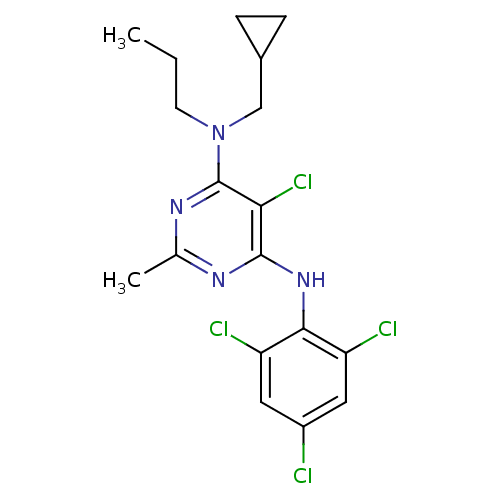 (5-Chloro-N-cyclopropylmethyl-2-methyl-N-propyl-N''...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135340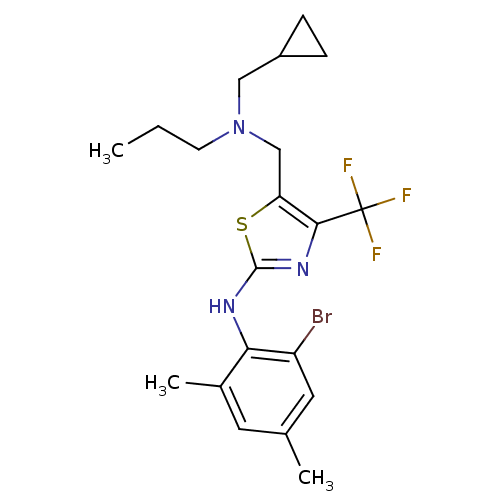 ((2-Bromo-4,6-dimethyl-phenyl)-{5-[(cyclopropylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135345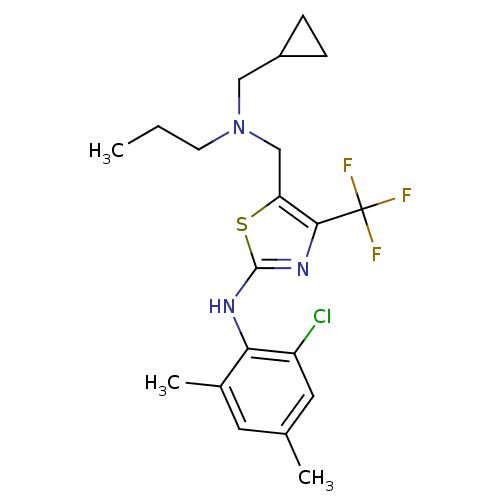 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135328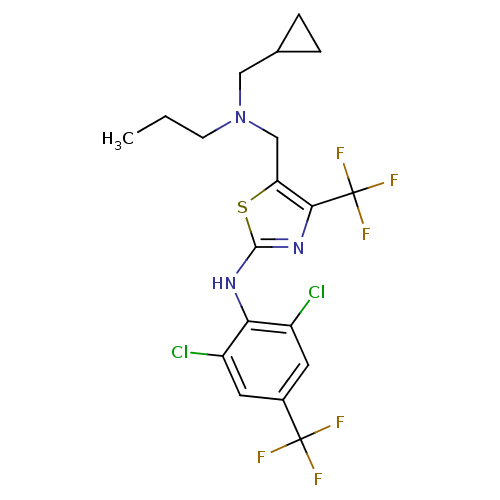 (CHEMBL131046 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135337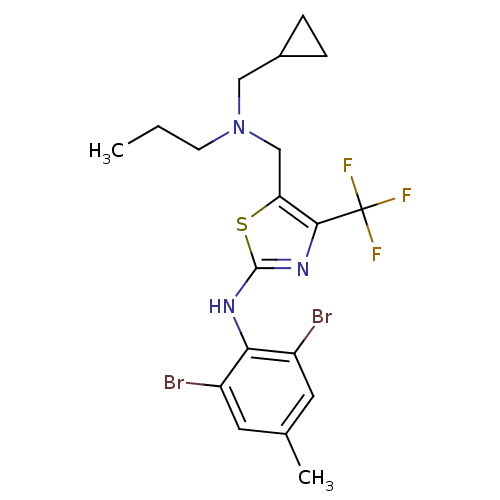 (CHEMBL131533 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135334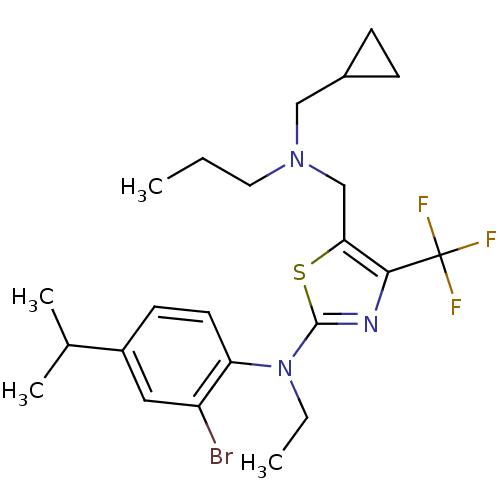 ((2-Bromo-4-isopropyl-phenyl)-{5-[(cyclopropylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135343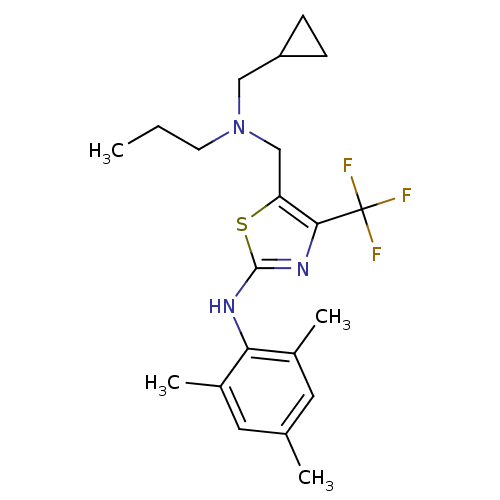 (CHEMBL128713 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135352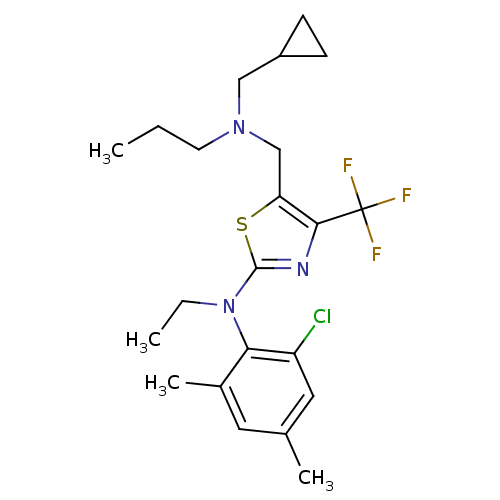 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135338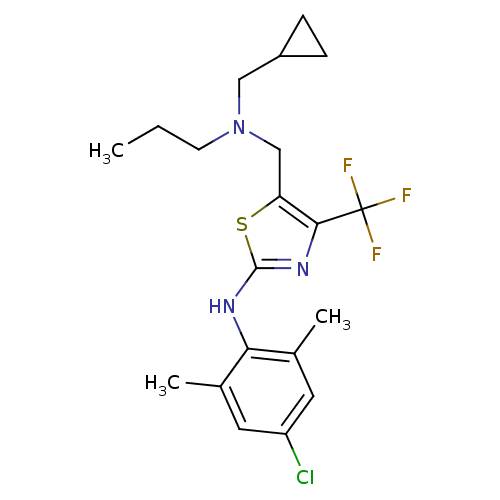 ((4-Chloro-2,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135336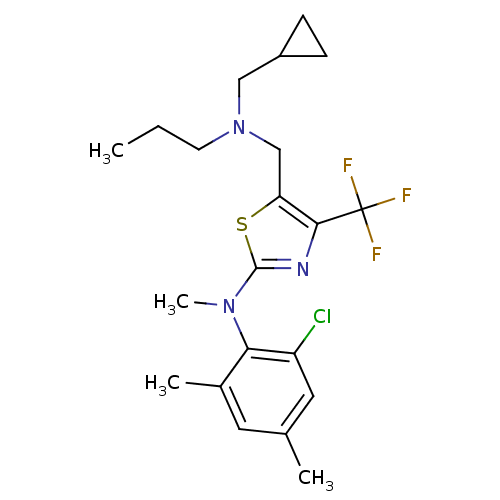 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135347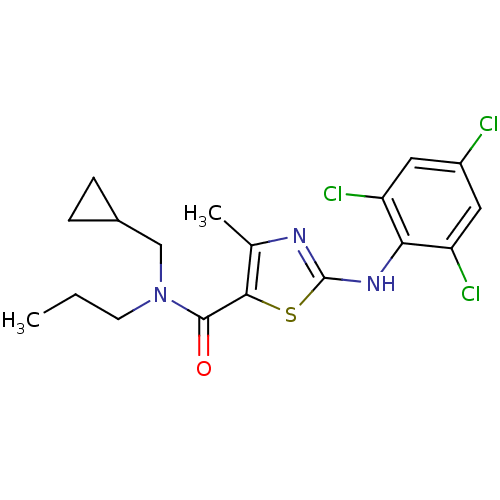 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135341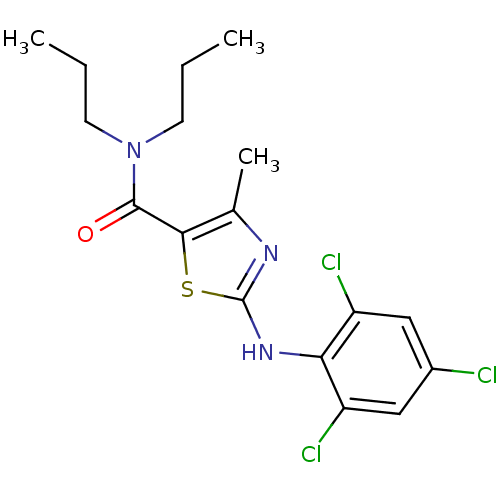 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135346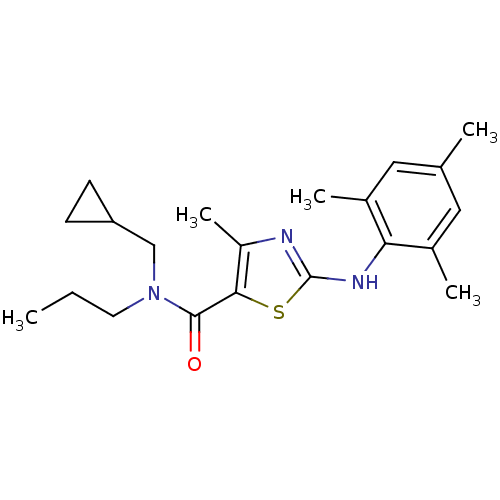 (4-Methyl-2-(2,4,6-trimethyl-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135344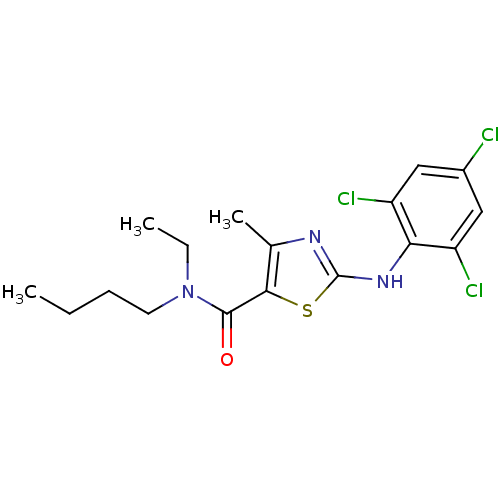 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135327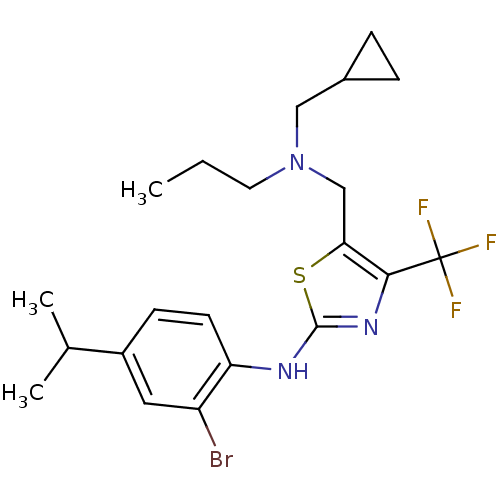 ((2-Bromo-4-isopropyl-phenyl)-{5-[(cyclopropylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135333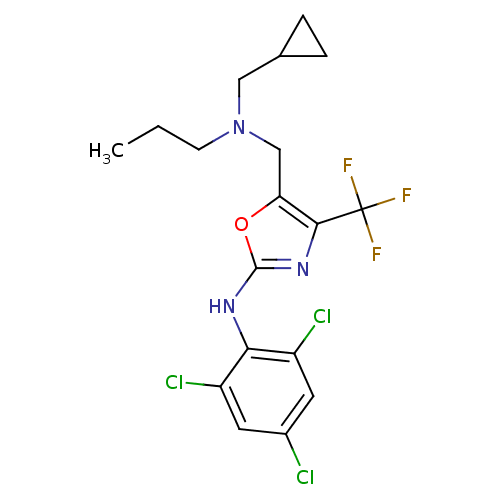 (CHEMBL128310 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135351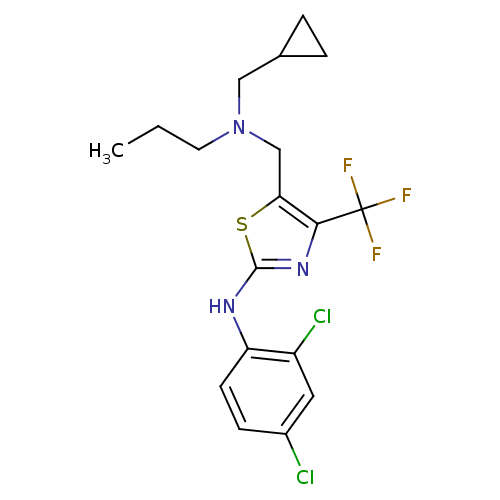 (CHEMBL338780 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135350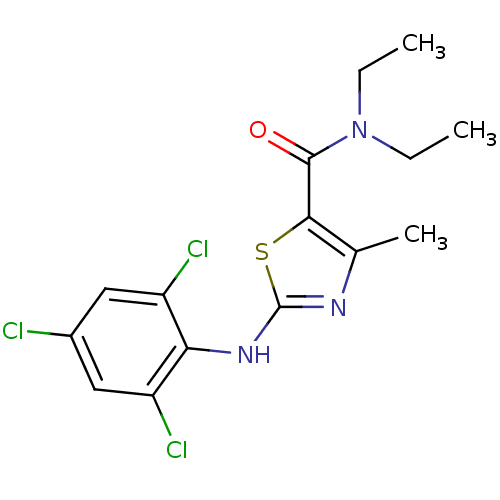 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135330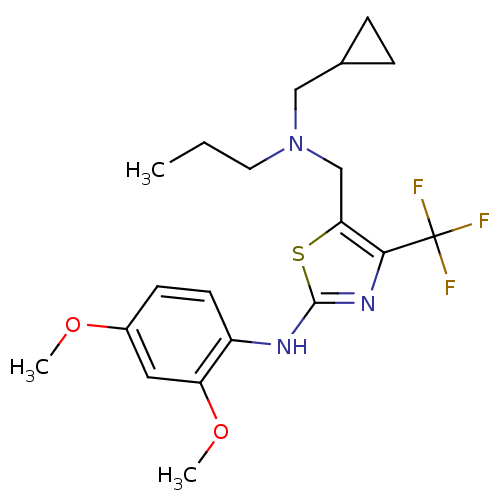 (CHEMBL127783 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135331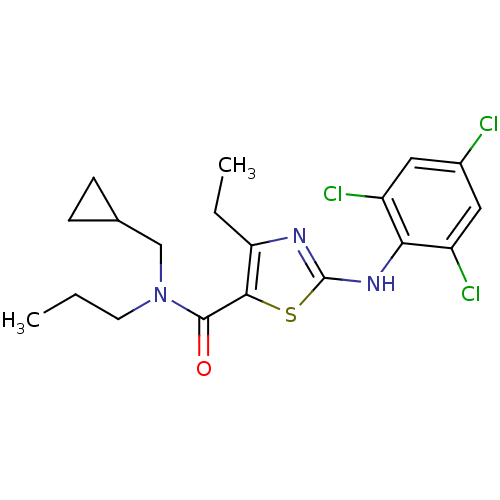 (4-Ethyl-2-(2,4,6-trichloro-phenylamino)-thiazole-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135348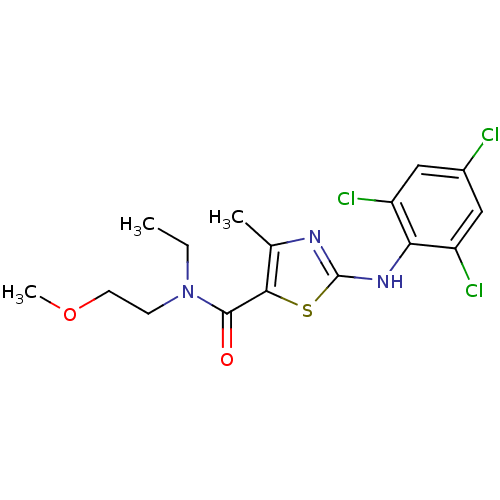 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135332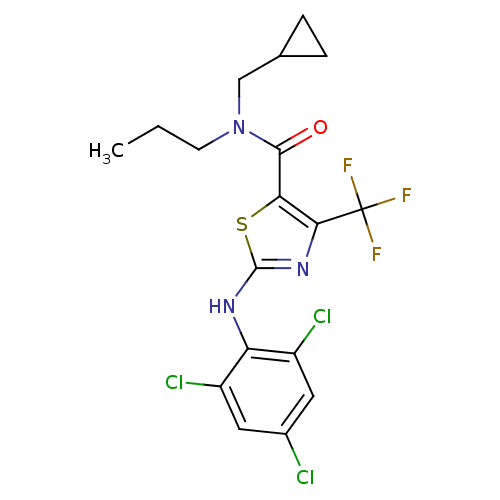 (2-(2,4,6-Trichloro-phenylamino)-4-trifluoromethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135342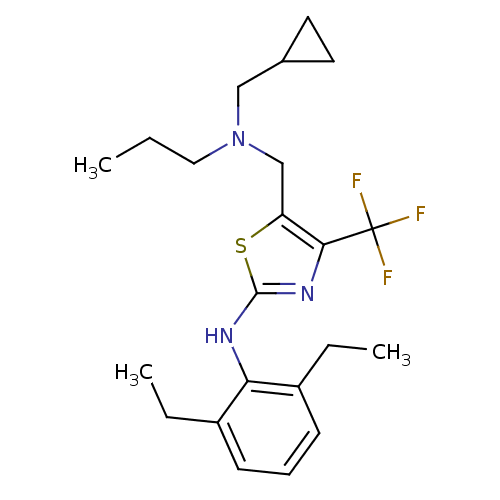 (CHEMBL340186 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135335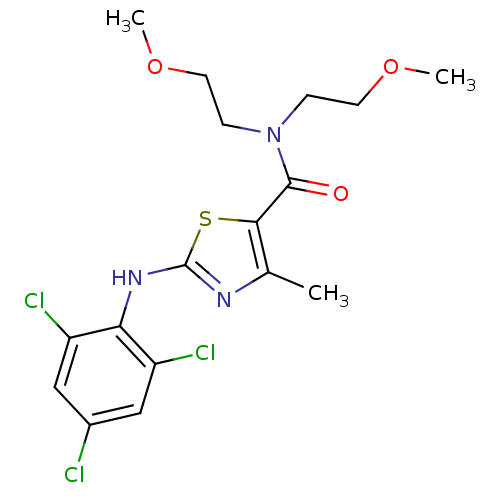 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50377170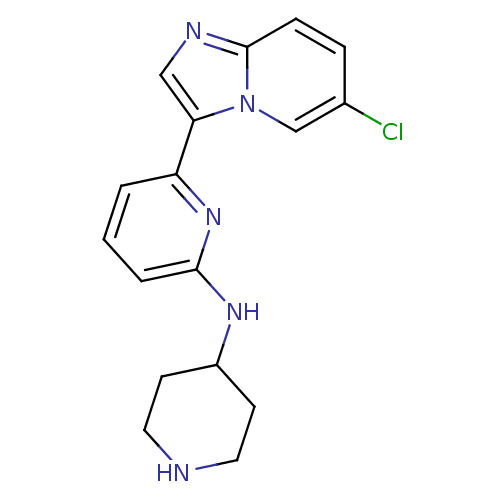 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of IRAK4 | Bioorg Med Chem Lett 18: 3656-60 (2008) Article DOI: 10.1016/j.bmcl.2008.04.042 BindingDB Entry DOI: 10.7270/Q2JH3N2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50377180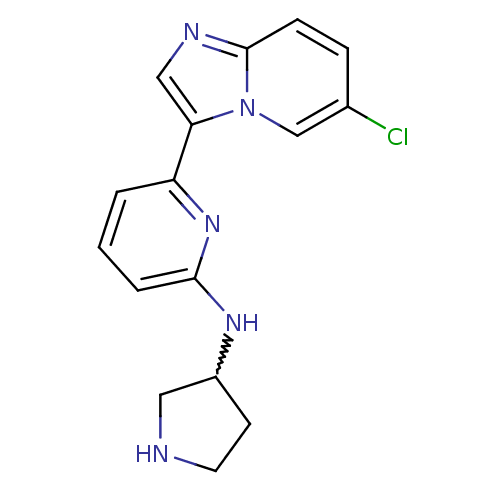 (CHEMBL401633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of IRAK4 | Bioorg Med Chem Lett 18: 3656-60 (2008) Article DOI: 10.1016/j.bmcl.2008.04.042 BindingDB Entry DOI: 10.7270/Q2JH3N2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311657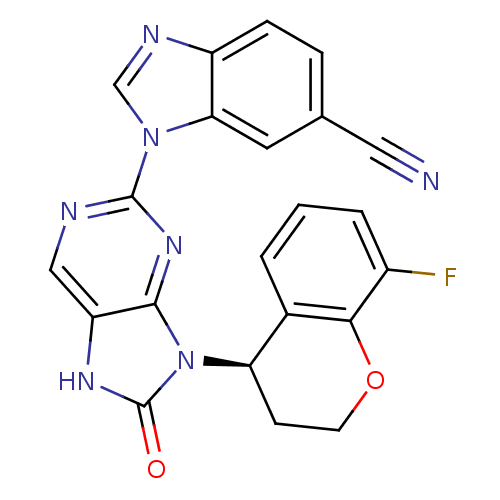 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50588566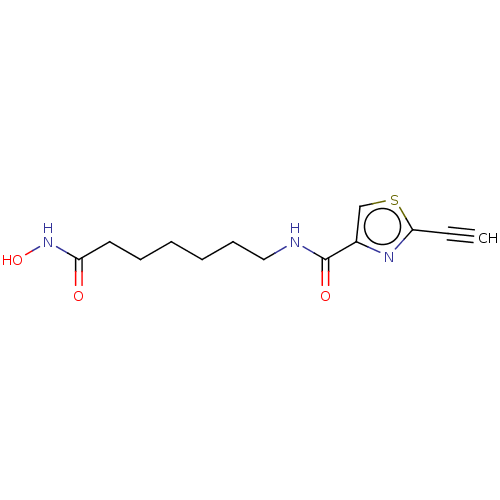 (CHEMBL5209294) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01276 BindingDB Entry DOI: 10.7270/Q2C53QTB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50377165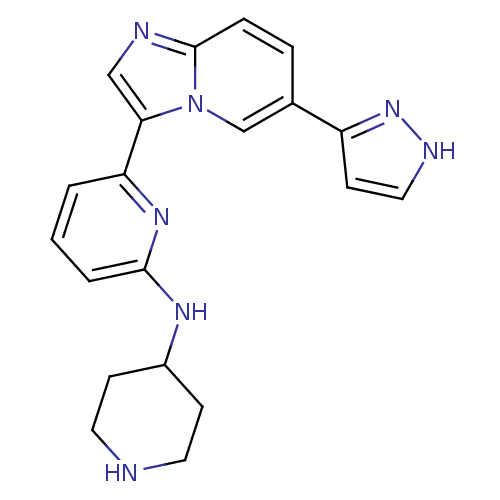 (CHEMBL255867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of IRAK4 | Bioorg Med Chem Lett 18: 3656-60 (2008) Article DOI: 10.1016/j.bmcl.2008.04.042 BindingDB Entry DOI: 10.7270/Q2JH3N2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164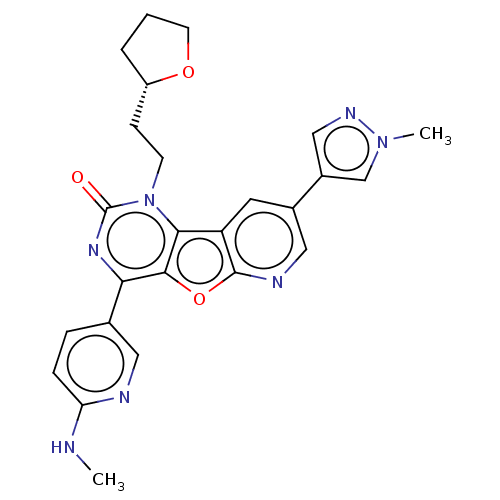 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156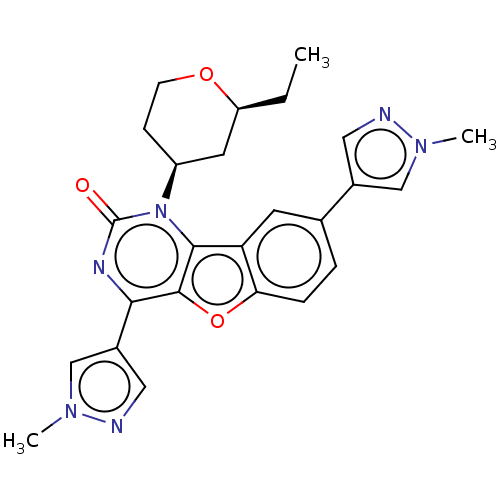 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155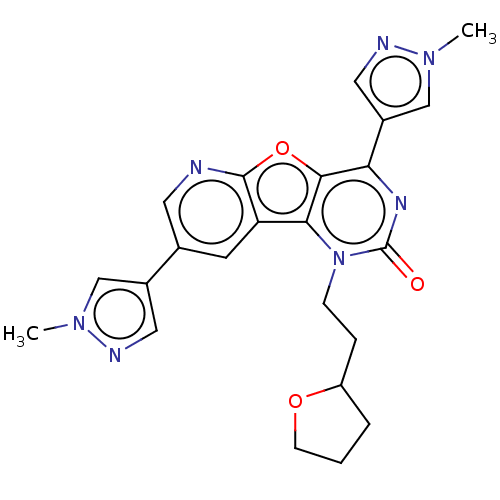 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50377175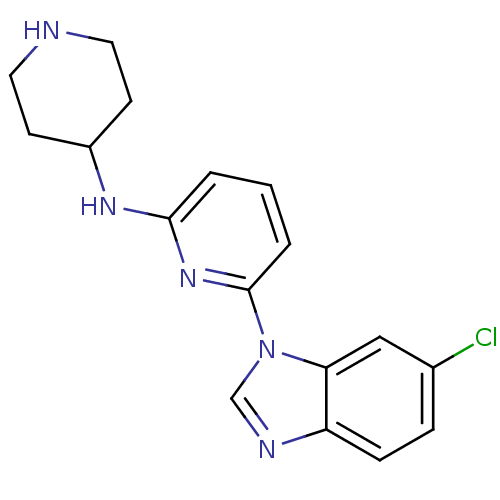 (CHEMBL436653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of IRAK4 | Bioorg Med Chem Lett 18: 3656-60 (2008) Article DOI: 10.1016/j.bmcl.2008.04.042 BindingDB Entry DOI: 10.7270/Q2JH3N2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311656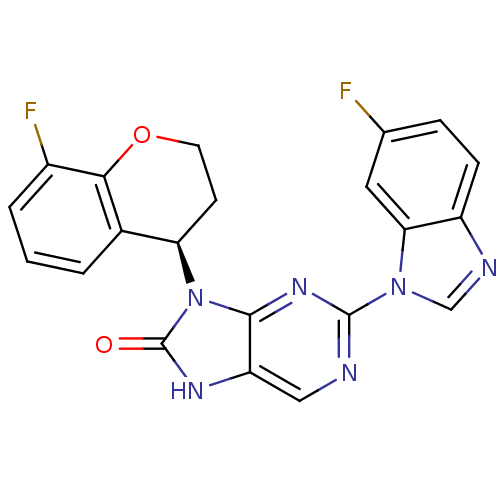 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176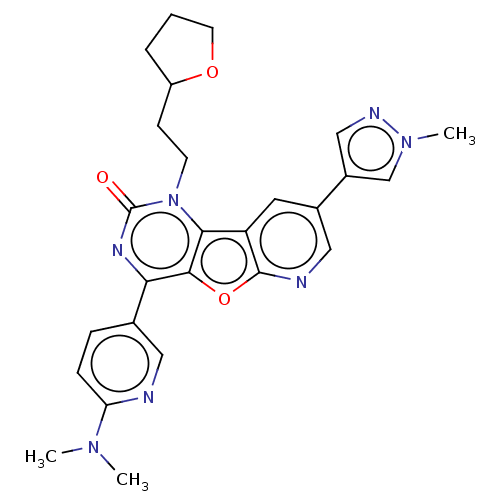 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492175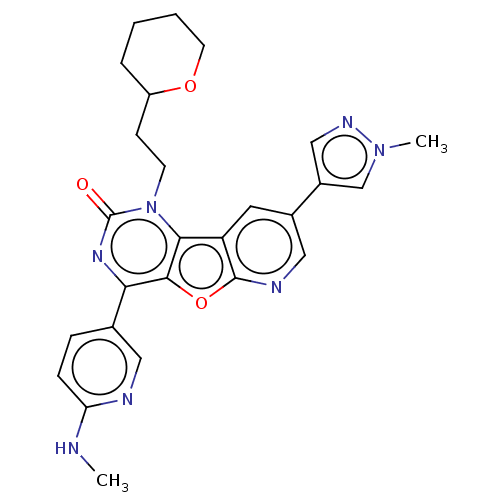 (CHEMBL2397583) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492163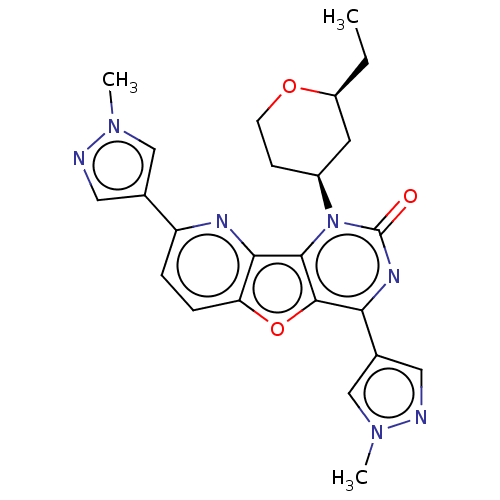 (CHEMBL2397557) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492154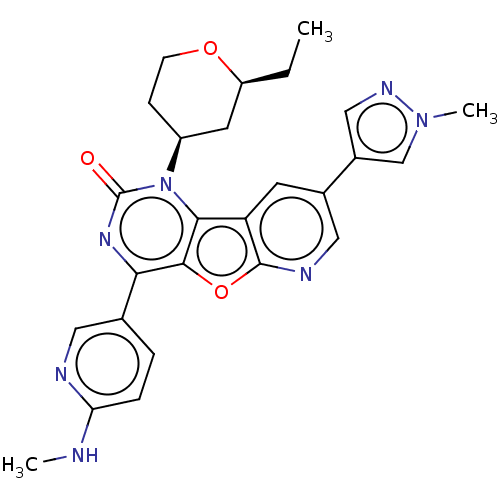 (CHEMBL2397572) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492168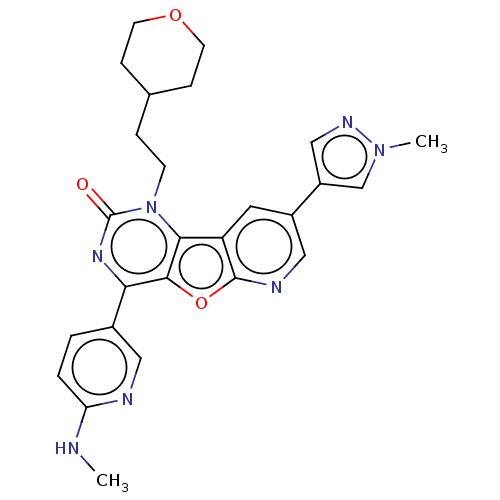 (CHEMBL2397584) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50377168 (CHEMBL255873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of IRAK4 | Bioorg Med Chem Lett 18: 3656-60 (2008) Article DOI: 10.1016/j.bmcl.2008.04.042 BindingDB Entry DOI: 10.7270/Q2JH3N2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50588561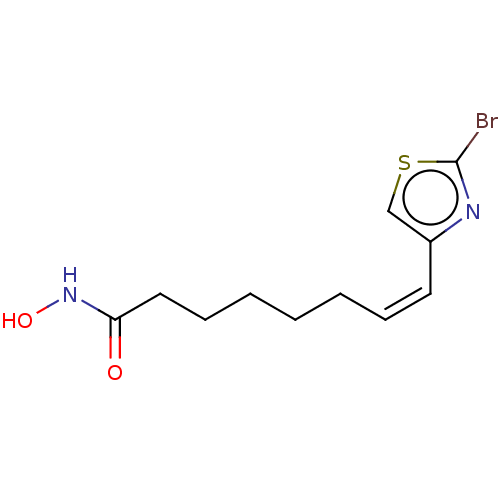 (CHEMBL5193020) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01276 BindingDB Entry DOI: 10.7270/Q2C53QTB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492153 (CHEMBL2397563) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492174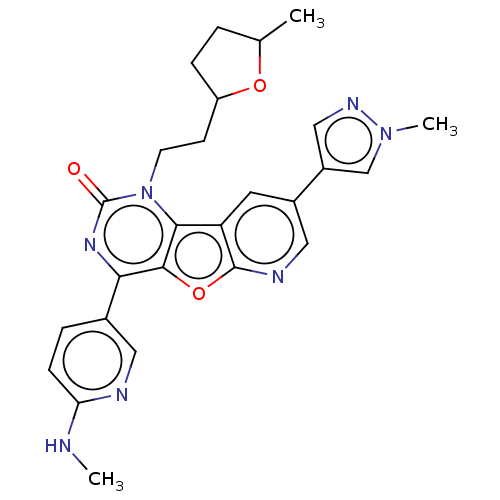 (CHEMBL2397586) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492169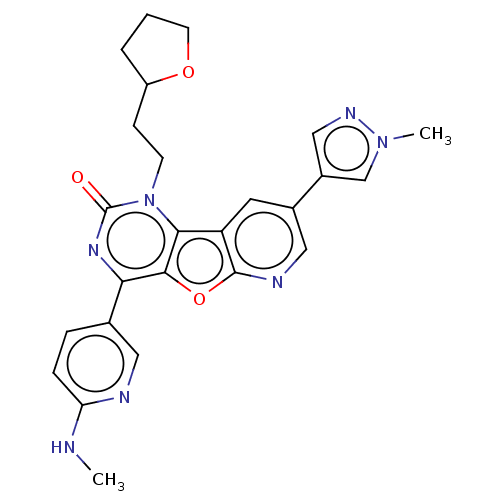 (CHEMBL2397582) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50211428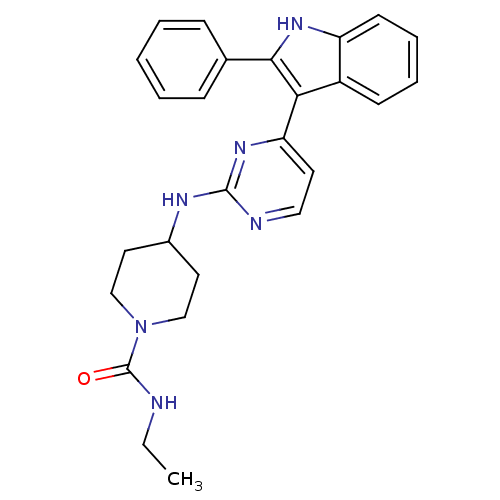 (CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of JNK3 | Bioorg Med Chem Lett 17: 3463-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.078 BindingDB Entry DOI: 10.7270/Q2959H7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50211428 (CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of JNK2 | Bioorg Med Chem Lett 17: 3463-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.078 BindingDB Entry DOI: 10.7270/Q2959H7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 450 total ) | Next | Last >> |