 Found 1801 hits with Last Name = 'lal' and Initial = 'a'
Found 1801 hits with Last Name = 'lal' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
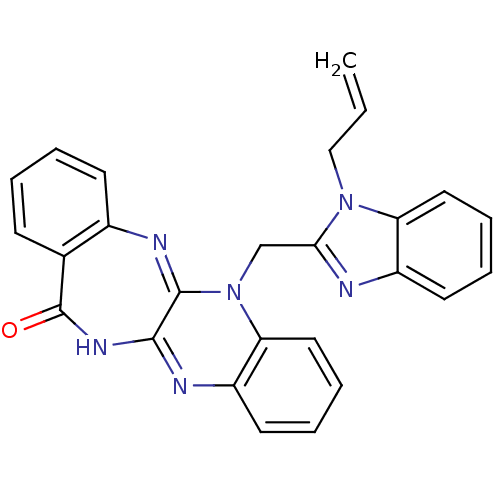
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
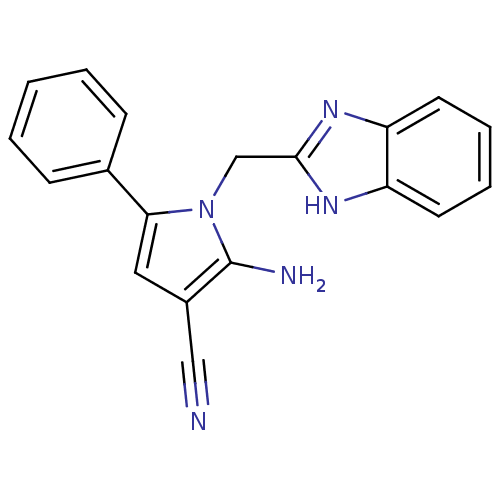
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
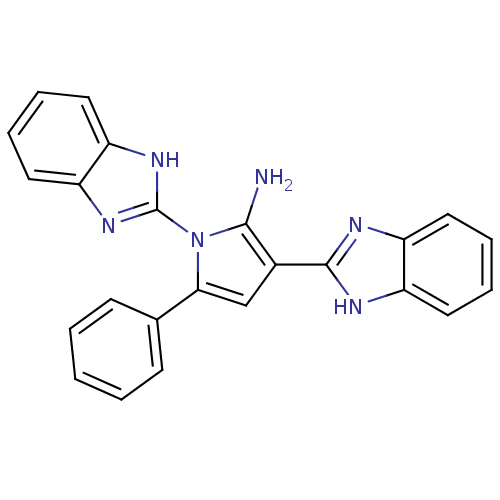
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
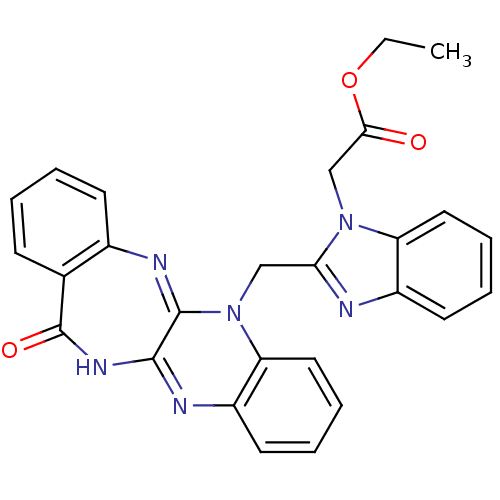
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
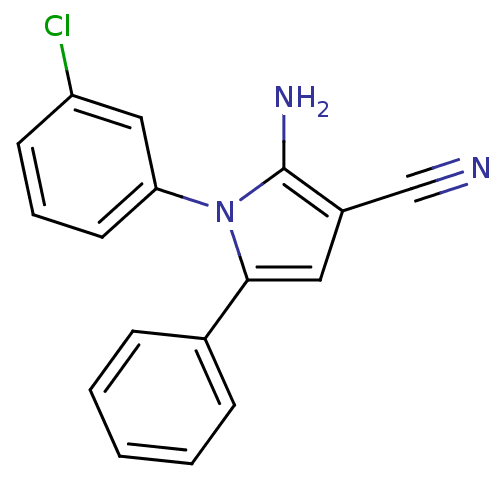
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
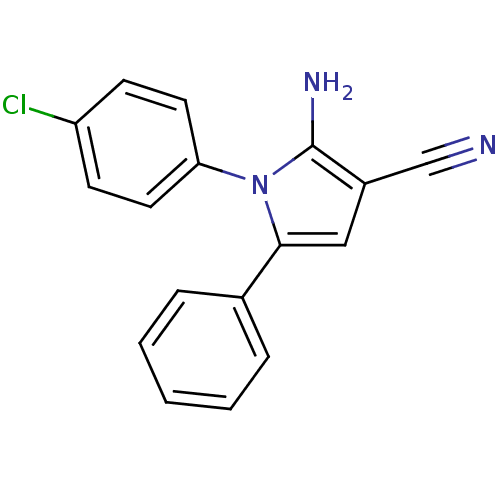
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
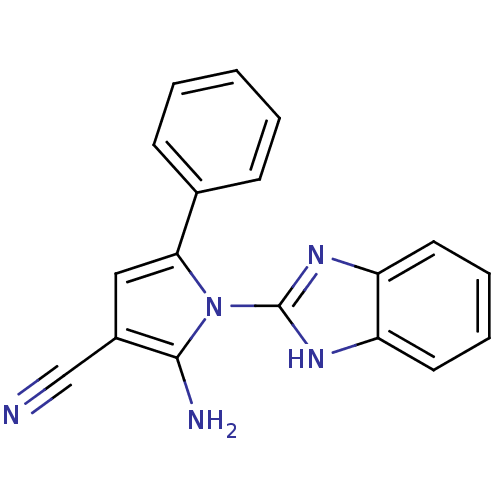
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
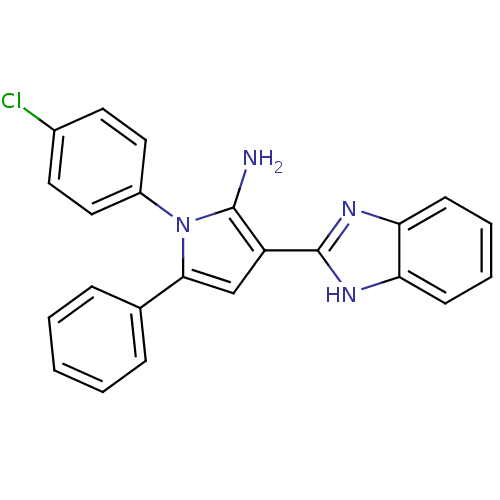
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
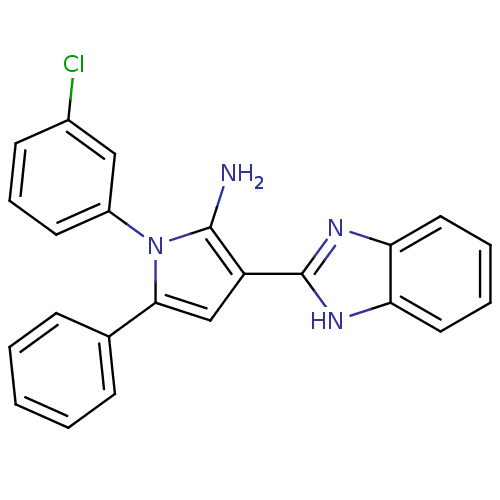
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
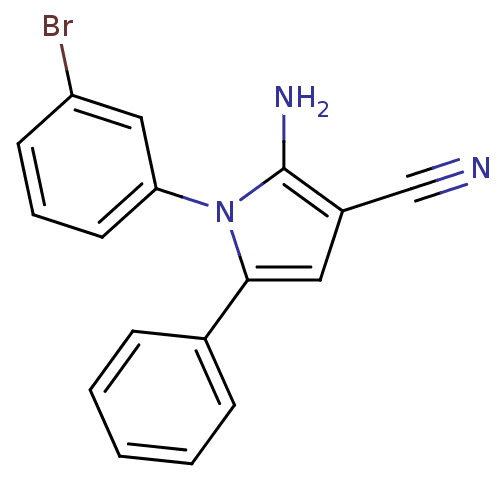
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
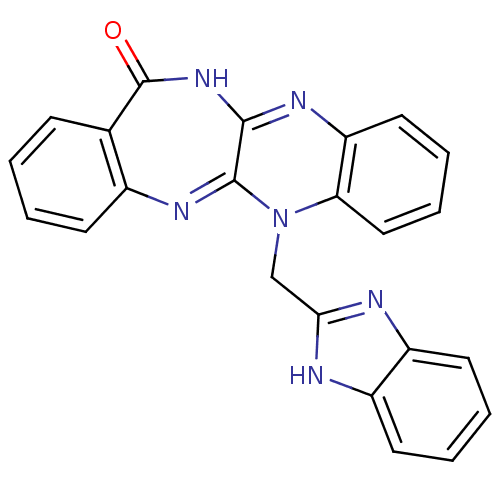
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
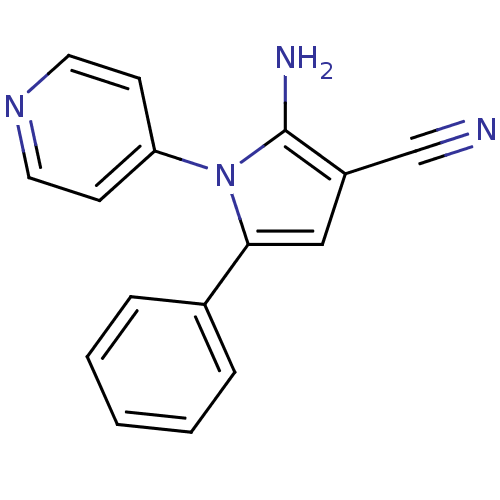
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130563
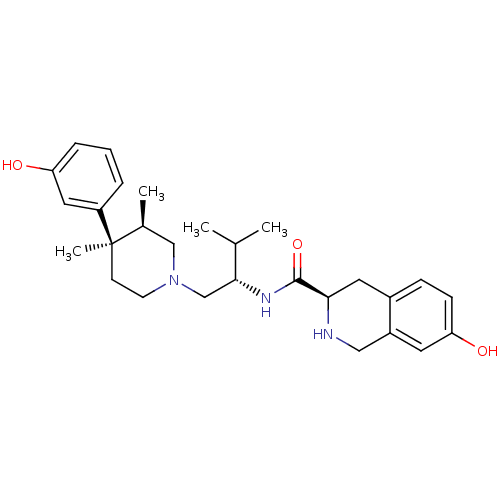
((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
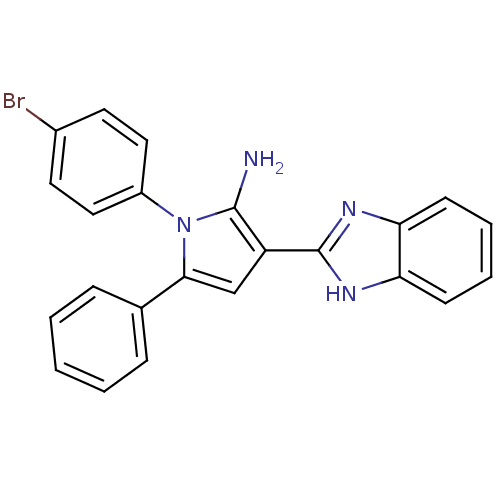
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
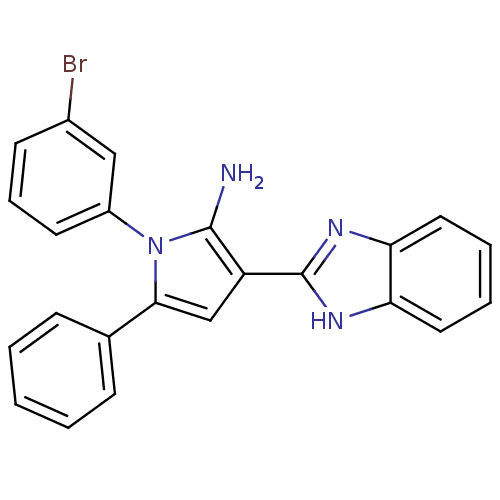
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50130563

((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50026614
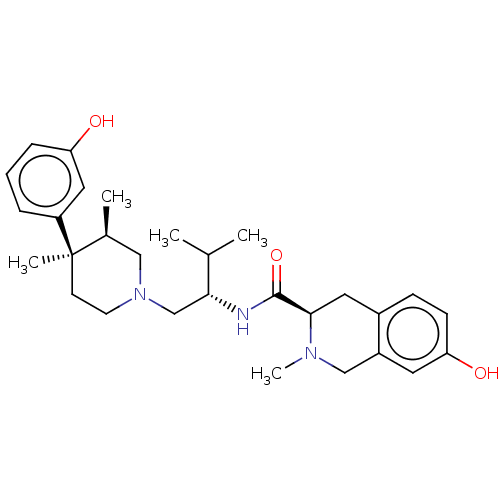
(CHEMBL575508)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1C |r| Show InChI InChI=1S/C29H41N3O3/c1-19(2)26(30-28(35)27-14-21-9-10-25(34)13-22(21)17-31(27)5)18-32-12-11-29(4,20(3)16-32)23-7-6-8-24(33)15-23/h6-10,13,15,19-20,26-27,33-34H,11-12,14,16-18H2,1-5H3,(H,30,35)/t20-,26+,27+,29+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50464462

(CHEMBL4283681)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC1C2Nc2ccc(CCNC(=N)CCCC)cc12)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C33H42N4O3/c1-2-3-4-27(34)35-13-11-19-7-9-24-22(15-19)23-17-33(39)26-16-21-8-10-25(38)30-28(21)32(33,31(40-30)29(23)36-24)12-14-37(26)18-20-5-6-20/h7-10,15,20,23,26,29,31,36,38-39H,2-6,11-14,16-18H2,1H3,(H2,34,35)/t23?,26-,29?,31+,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775
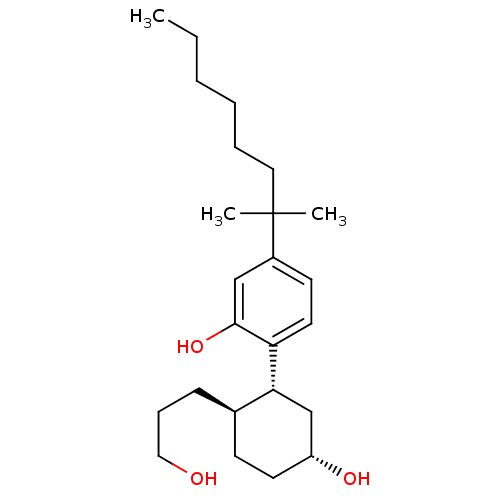
(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay |
Eur J Med Chem 143: 983-996 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.043
BindingDB Entry DOI: 10.7270/Q2D50QN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
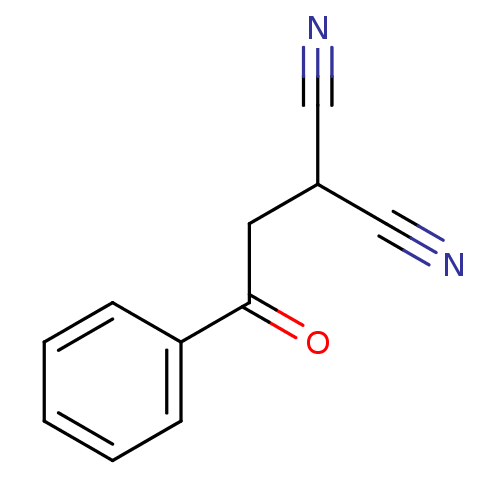
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50412340
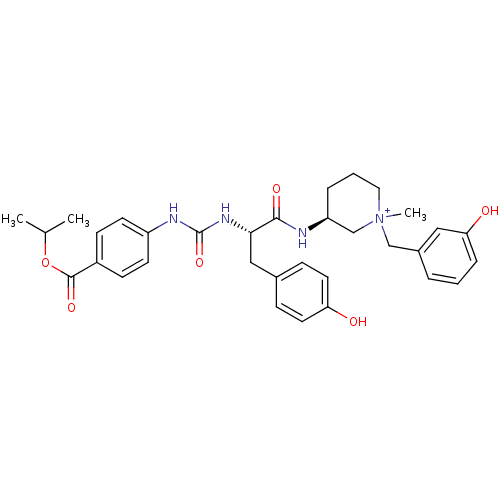
(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method |
Eur J Med Chem 143: 983-996 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.043
BindingDB Entry DOI: 10.7270/Q2D50QN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method |
Eur J Med Chem 143: 983-996 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.043
BindingDB Entry DOI: 10.7270/Q2D50QN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay |
Eur J Med Chem 143: 983-996 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.043
BindingDB Entry DOI: 10.7270/Q2D50QN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402368
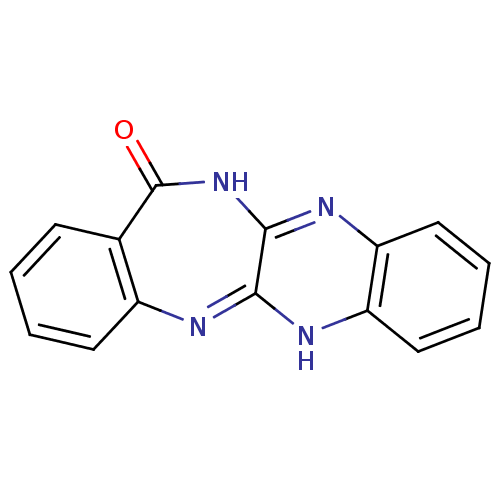
(CHEMBL2206682)Show InChI InChI=1S/C15H10N4O/c20-15-9-5-1-2-6-10(9)16-13-14(19-15)18-12-8-4-3-7-11(12)17-13/h1-8H,(H,16,17)(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580098

(CHEMBL5075948)Show SMILES Cc1cn([C@H]2C[C@H]([C@@H](CO)O2)n2cc(COC(=O)c3ccc(cc3)S(N)(=O)=O)nn2)c(=O)[nH]c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070
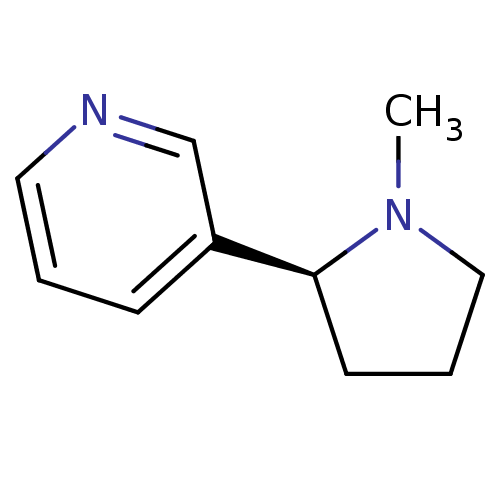
(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I] alpha bungarotoxin from human neuronal alpha4beta2 nAChR expressed in human SH-SY5Y cell membrane measured after 120 mins by T... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112403
BindingDB Entry DOI: 10.7270/Q2CF9TV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50130563

((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50012152

(CHEMBL3264441)Show SMILES CCOC(=O)C(CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)OC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H36N2O6/c1-4-35-27(34)25(36-26(33)24-13-19-8-9-23(32)12-20(19)15-29-24)17-30-11-10-28(3,18(2)16-30)21-6-5-7-22(31)14-21/h5-9,12,14,18,24-25,29,31-32H,4,10-11,13,15-17H2,1-3H3/t18-,24+,25?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 7 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50580101
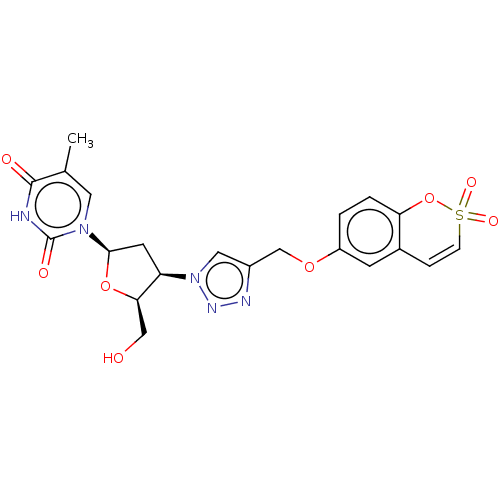
(CHEMBL5081179)Show SMILES Cc1cn([C@H]2C[C@H]([C@@H](CO)O2)n2cc(COc3ccc4OS(=O)(=O)C=Cc4c3)nn2)c(=O)[nH]c1=O |r,c:25| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 12 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50412340

(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50464462

(CHEMBL4283681)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC1C2Nc2ccc(CCNC(=N)CCCC)cc12)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C33H42N4O3/c1-2-3-4-27(34)35-13-11-19-7-9-24-22(15-19)23-17-33(39)26-16-21-8-10-25(38)30-28(21)32(33,31(40-30)29(23)36-24)12-14-37(26)18-20-5-6-20/h7-10,15,20,23,26,29,31,36,38-39H,2-6,11-14,16-18H2,1H3,(H2,34,35)/t23?,26-,29?,31+,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50580100
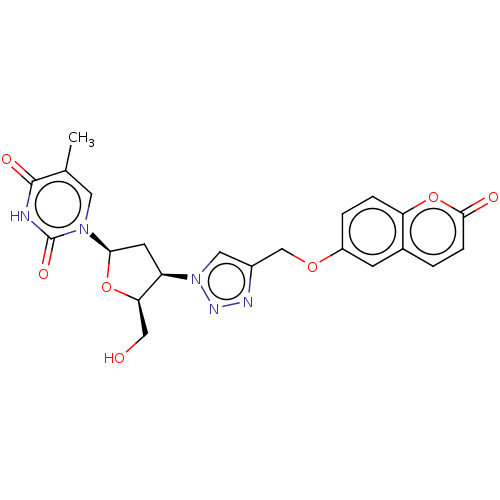
(CHEMBL5089227)Show SMILES Cc1cn([C@H]2C[C@H]([C@@H](CO)O2)n2cc(COc3ccc4oc(=O)ccc4c3)nn2)c(=O)[nH]c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 12 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580098

(CHEMBL5075948)Show SMILES Cc1cn([C@H]2C[C@H]([C@@H](CO)O2)n2cc(COC(=O)c3ccc(cc3)S(N)(=O)=O)nn2)c(=O)[nH]c1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 9 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50442103
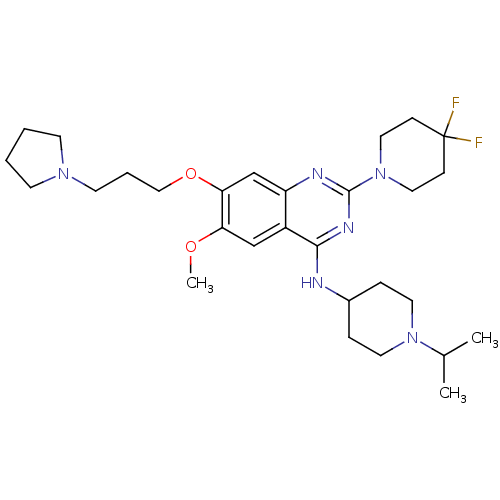
(CHEMBL2441082)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C29H44F2N6O2/c1-21(2)36-14-7-22(8-15-36)32-27-23-19-25(38-3)26(39-18-6-13-35-11-4-5-12-35)20-24(23)33-28(34-27)37-16-9-29(30,31)10-17-37/h19-22H,4-18H2,1-3H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of lysine methyltransferase G9a (unknown origin) using SAM as substrate by Michaelis-Menten kinetic assay |
J Med Chem 56: 8931-42 (2013)
Article DOI: 10.1021/jm401480r
BindingDB Entry DOI: 10.7270/Q2NZ892T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50464462

(CHEMBL4283681)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC1C2Nc2ccc(CCNC(=N)CCCC)cc12)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C33H42N4O3/c1-2-3-4-27(34)35-13-11-19-7-9-24-22(15-19)23-17-33(39)26-16-21-8-10-25(38)30-28(21)32(33,31(40-30)29(23)36-24)12-14-37(26)18-20-5-6-20/h7-10,15,20,23,26,29,31,36,38-39H,2-6,11-14,16-18H2,1H3,(H2,34,35)/t23?,26-,29?,31+,32+,33-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CA12 by stopped-flow carbon dioxide hydration assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112439
BindingDB Entry DOI: 10.7270/Q2057KNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 12 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703
(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703

(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703

(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580099
(CHEMBL5078071)Show SMILES Cc1cn([C@H]2C[C@H]([C@@H](CO)O2)n2cc(CNC(=O)Nc3cccc(c3)S(N)(=O)=O)nn2)c(=O)[nH]c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00756
BindingDB Entry DOI: 10.7270/Q2V4103G |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data