

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048691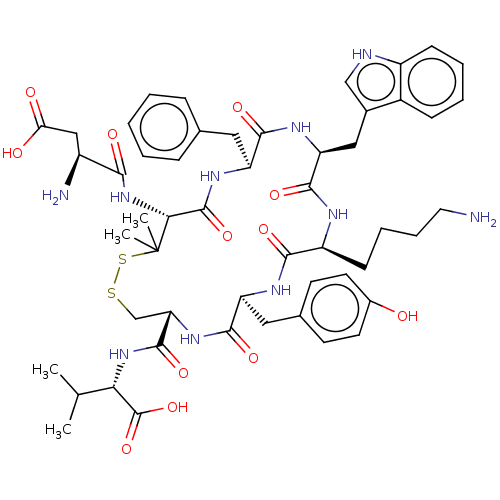 (CHEMBL3315139) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50320463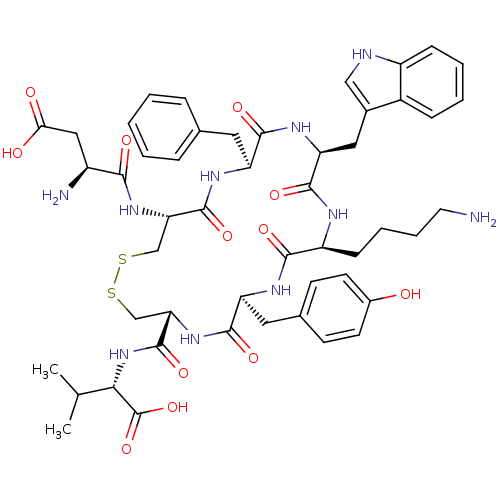 (CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048696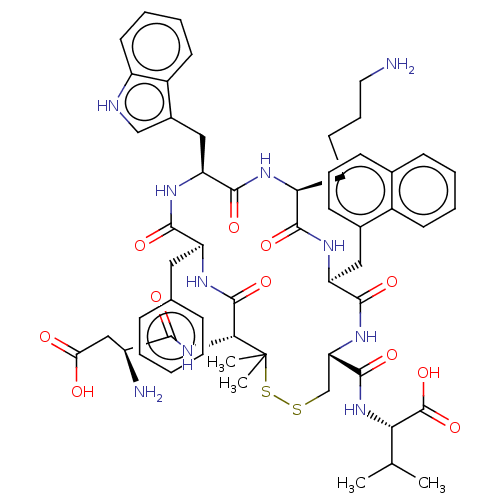 (CHEMBL3315142) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048695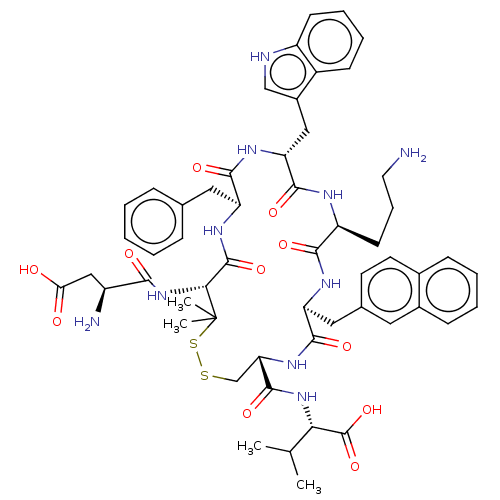 (CHEMBL3315141) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048701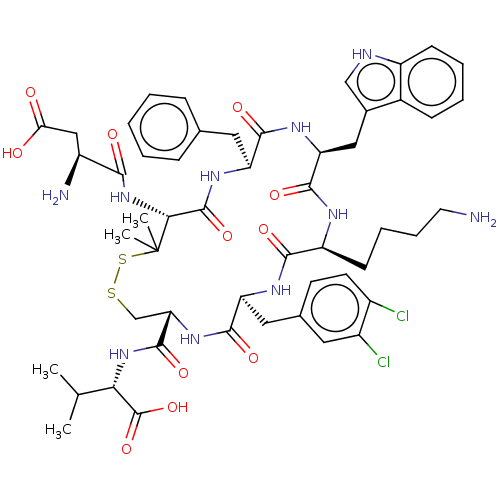 (CHEMBL3315148) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413784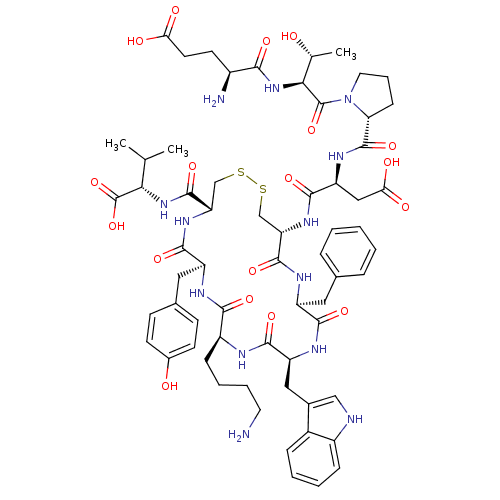 (UROTENSIN-II) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048700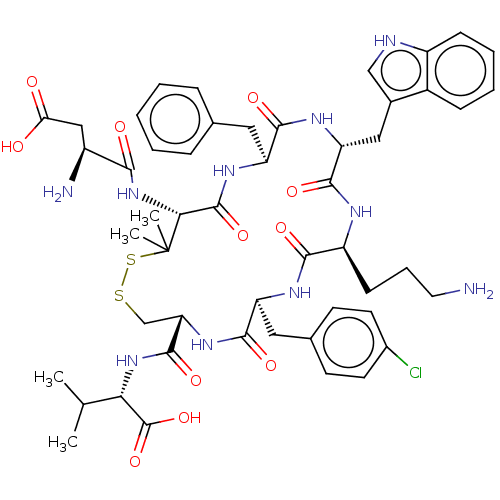 (CHEMBL3315147) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048699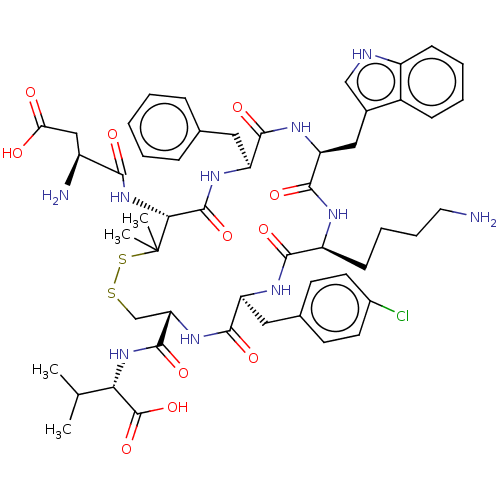 (CHEMBL3315146) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048697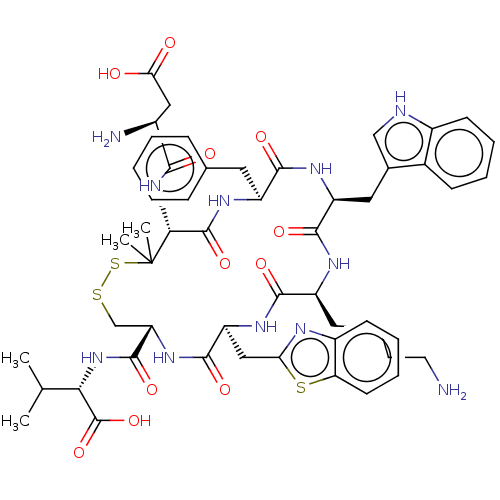 (CHEMBL3315144) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026764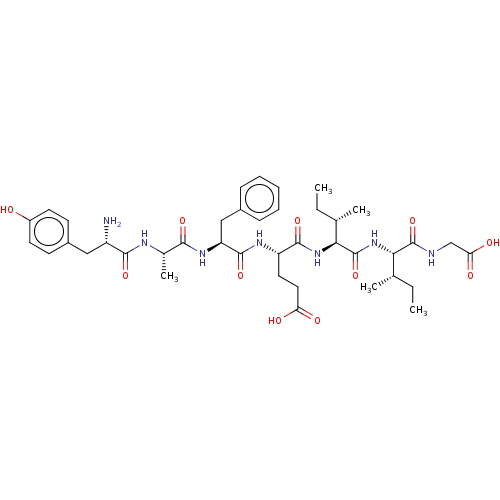 (CHEMBL3331509) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]Ile5,6deltorphin II from delta opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition b... | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048705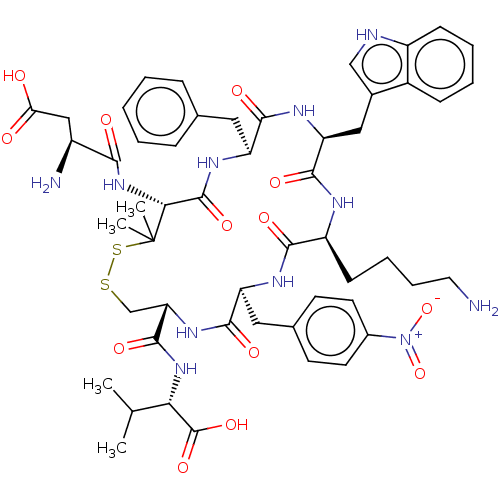 (CHEMBL3315152) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048703 (CHEMBL3315150) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026762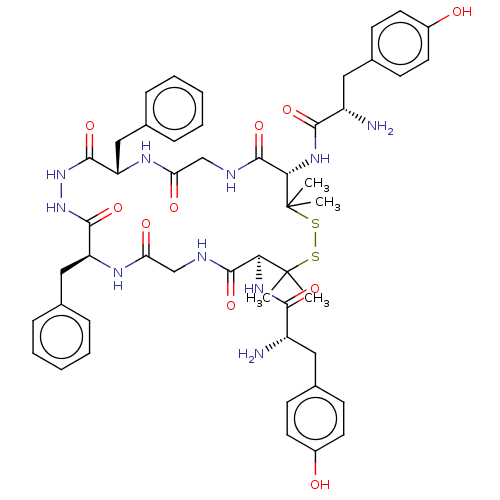 (CHEMBL3331510) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048692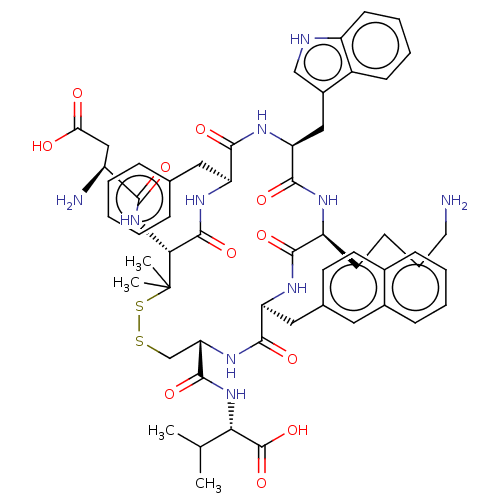 (CHEMBL3315140) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048702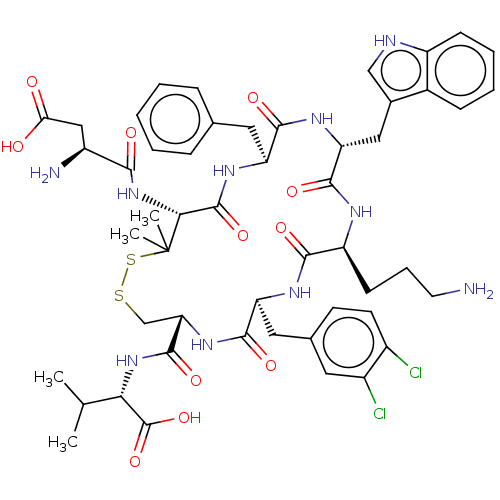 (CHEMBL3315149) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21014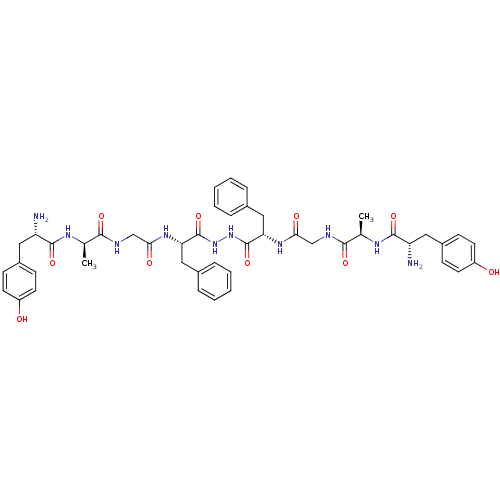 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50411333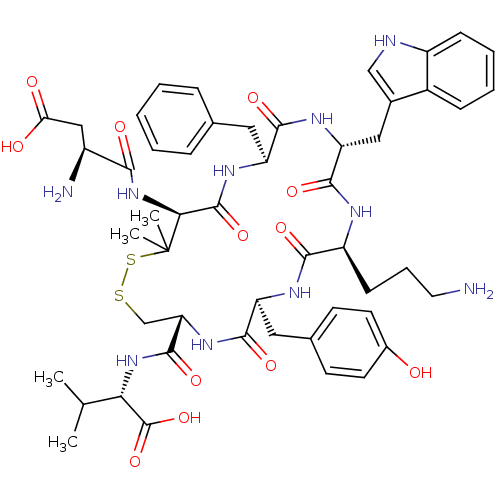 (URANTIDE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026762 (CHEMBL3331510) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]Ile5,6deltorphin II from delta opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition b... | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50381677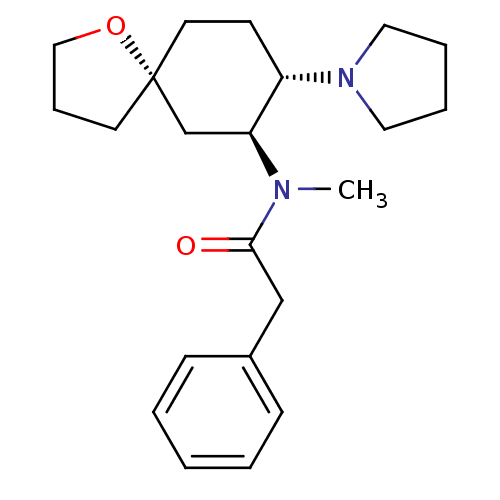 (CHEMBL1256748 | U-69593) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048690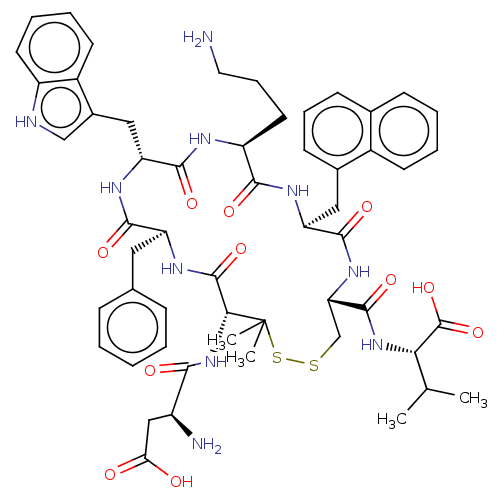 (CHEMBL3315143) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048704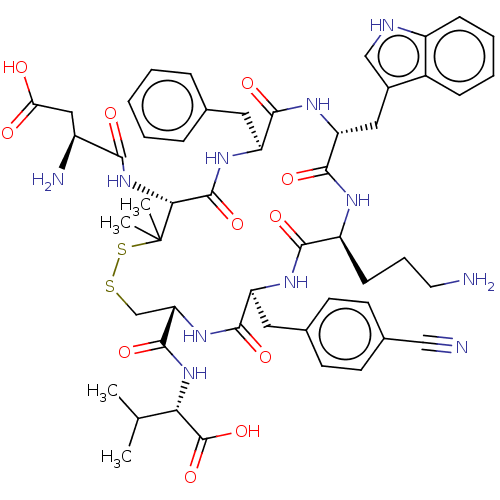 (CHEMBL3315151) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048698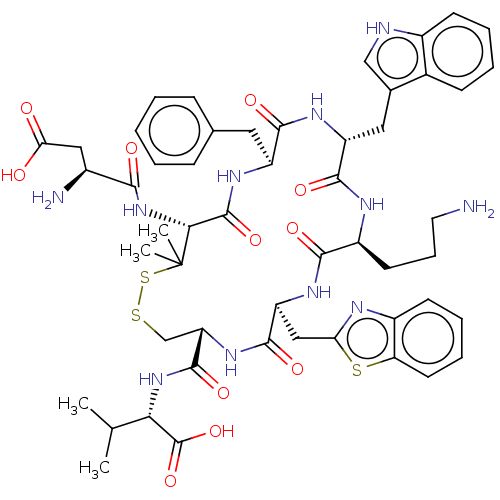 (CHEMBL3315145) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]Ile5,6deltorphin II from delta opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition b... | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048706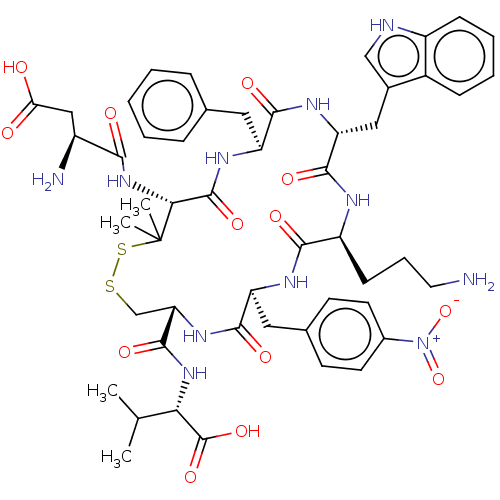 (CHEMBL3315153) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Binding affinity to 6xHis-taged MDM2 (unknown origin) expressed in Escherichia coli Gold (DE3) assessed as inhibition constant | ACS Med Chem Lett 11: 1047-1053 (2020) Article DOI: 10.1021/acsmedchemlett.9b00620 BindingDB Entry DOI: 10.7270/Q20K2D3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026762 (CHEMBL3331510) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 283 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453125 (CHEMBL4214826) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453133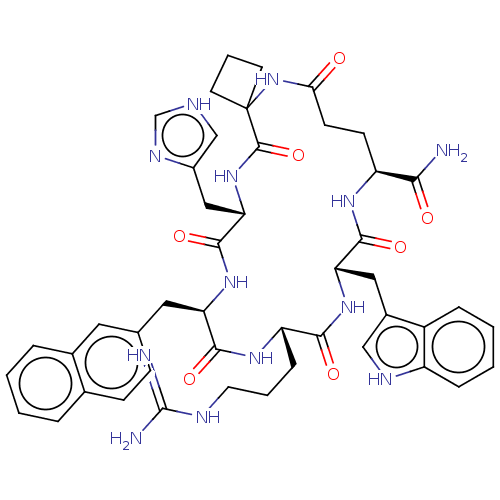 (CHEMBL4211275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453126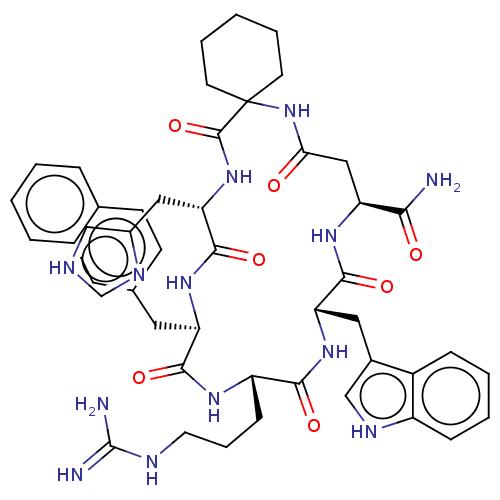 (CHEMBL4208874) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453137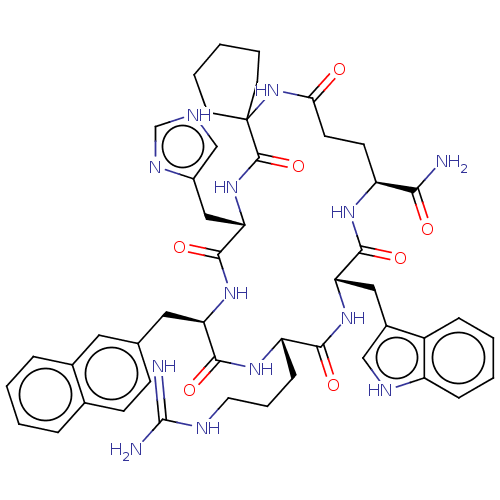 (CHEMBL4217528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453138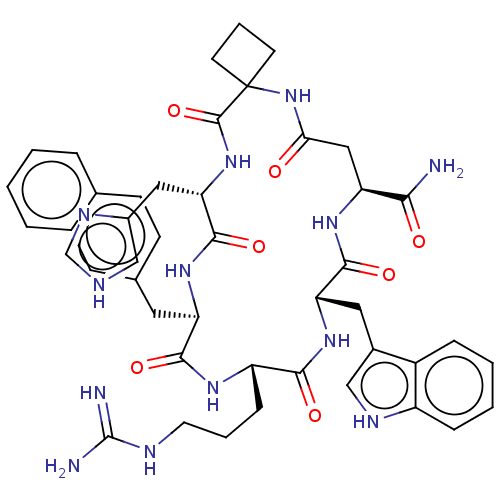 (CHEMBL4212209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50453132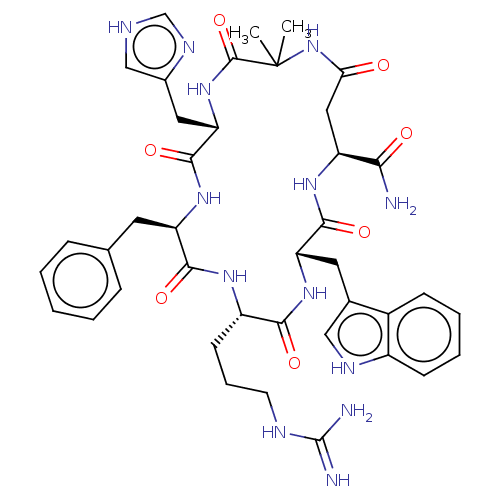 (CHEMBL4207725) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50026762 (CHEMBL3331510) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-induced smooth muscle contraction | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of electrically-induce... | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453135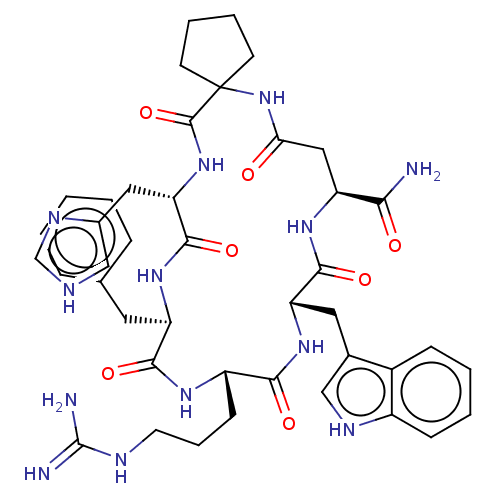 (CHEMBL4204975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453130 (CHEMBL4206406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50453128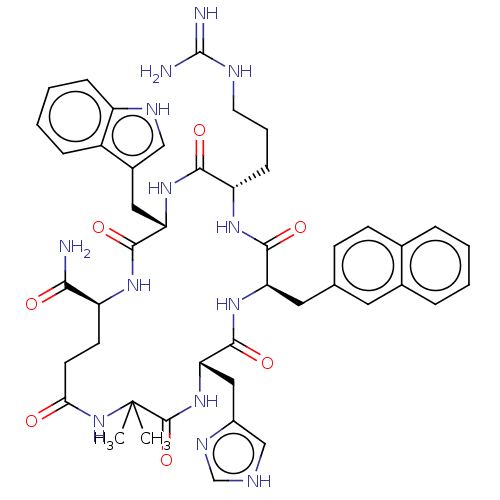 (CHEMBL4207858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453131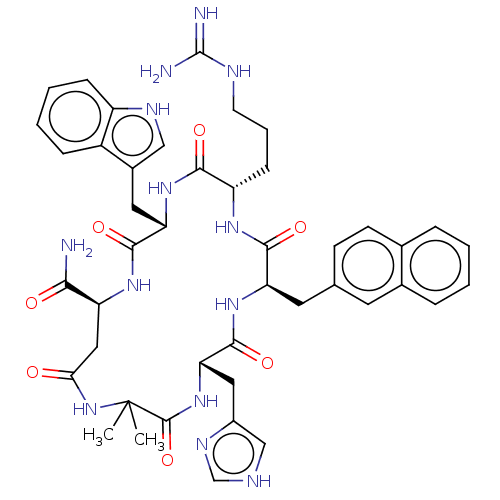 (CHEMBL4212629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453134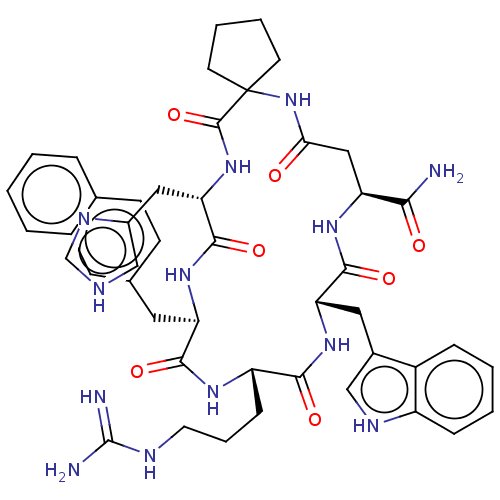 (CHEMBL4216654) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50453137 (CHEMBL4217528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50453132 (CHEMBL4207725) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50026762 (CHEMBL3331510) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of electrically-induce... | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50453133 (CHEMBL4211275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in HEK293 cells after 40 mins by gamma counting method | J Med Chem 61: 4263-4269 (2018) Article DOI: 10.1021/acs.jmedchem.8b00488 BindingDB Entry DOI: 10.7270/Q22J6FF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-induced smooth muscle contraction | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |