

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21216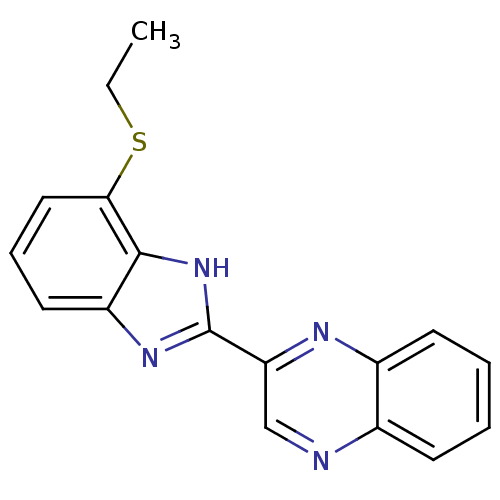 (2-[4-(ethylsulfanyl)-1H-1,3-benzodiazol-2-yl]quino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432208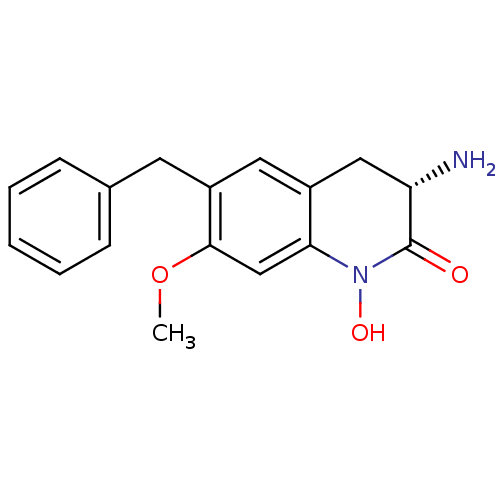 (CHEMBL2347110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426340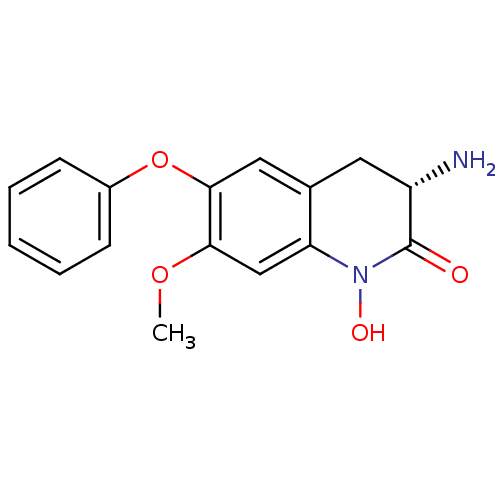 (CHEMBL2321943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107730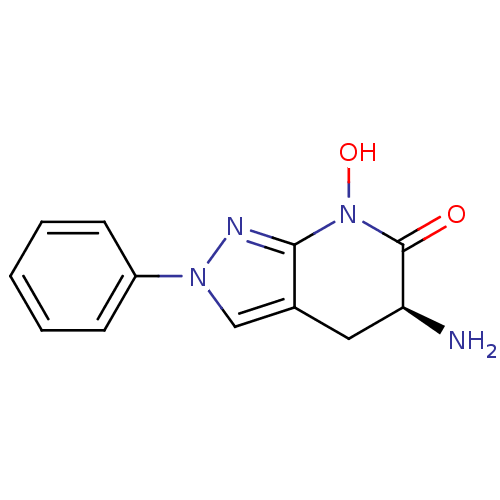 (CHEMBL2347108 | US8933095, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50179306 (2-(4-(methylthio)-1H-benzo[d]imidazol-2-yl)quinoxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309987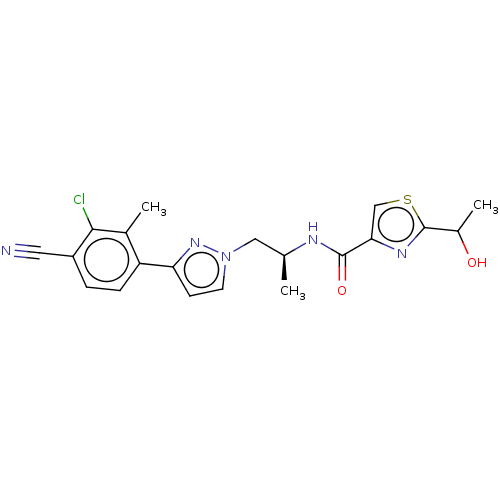 (N-((S)-1-(3-(3-chloro-4-cyano-2-methylphenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508526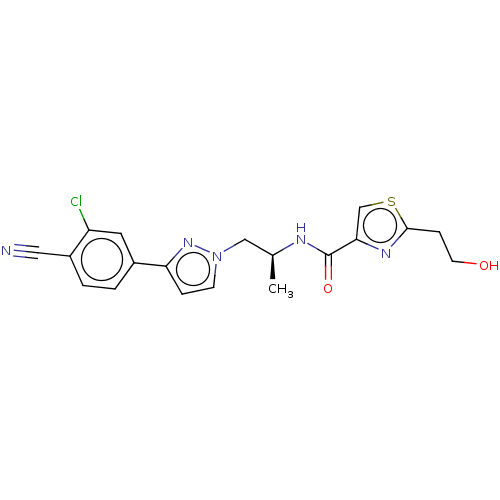 (N-((S)-1-(3-(3-chloro-4-cyano-2-methylphenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50179304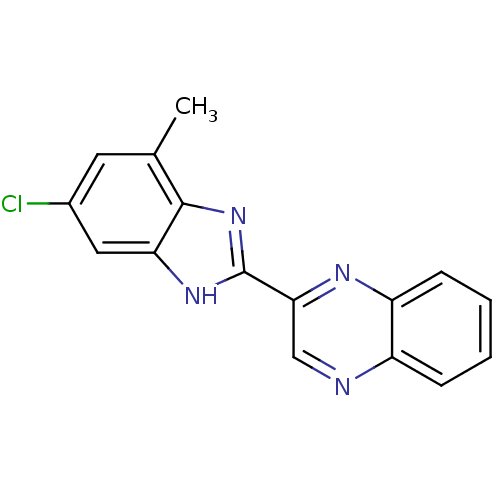 (2-(6-chloro-4-methyl-1H-benzo[d]imidazol-2-yl)quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309973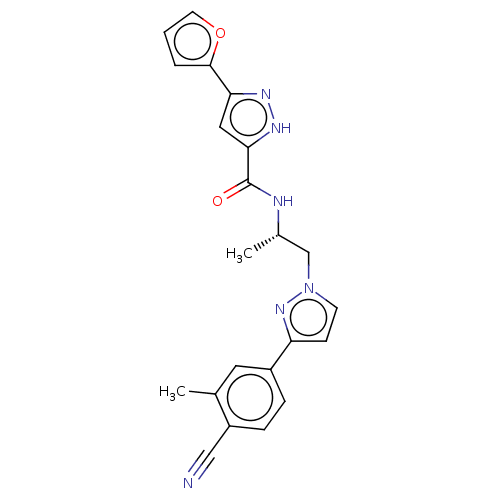 ((S)-N-(1-(3-(4-cyano-3-methylphenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309973 ((S)-N-(1-(3-(4-cyano-3-methylphenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426341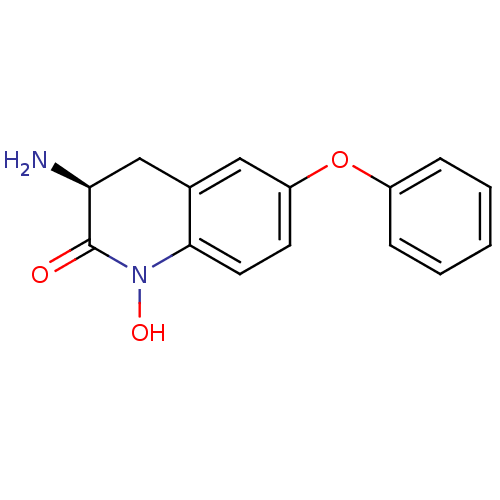 (CHEMBL2321944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225657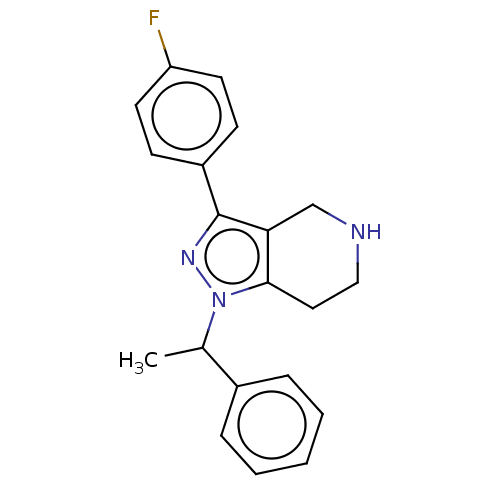 (CHEMBL173570) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386310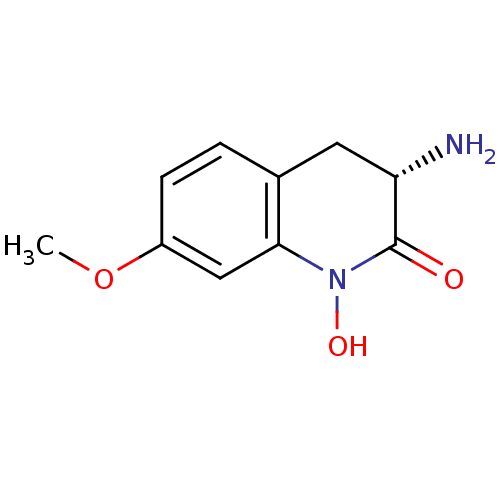 (CHEMBL2049092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309982 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309982 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225567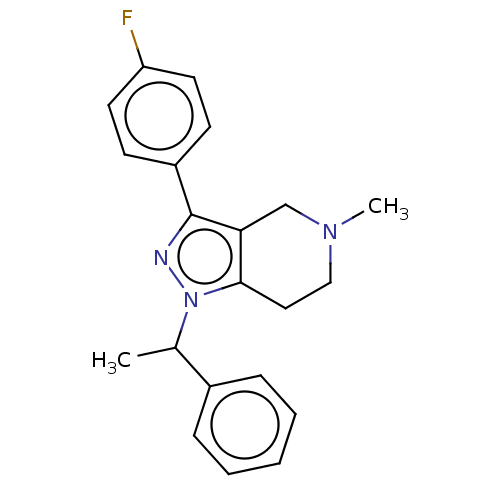 (CHEMBL559369) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225661 (CHEMBL173988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Rattus norvegicus (Rat)) | BDBM50185264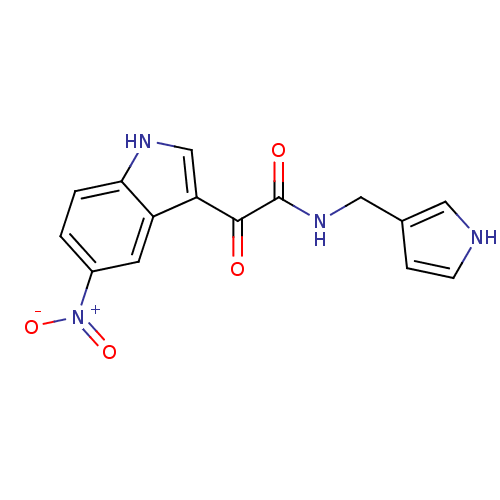 (CHEMBL204841 | N-((1H-pyrrol-3-yl)methyl)-2-(5-nit...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]flumazenil from rat GABA-Aalpha1 receptor plus beta-2-gamma-2 in HEK293 cells | J Med Chem 49: 2489-95 (2006) Article DOI: 10.1021/jm0511841 BindingDB Entry DOI: 10.7270/Q25B038F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50179293 (2-(4,6-dichloro-1H-benzo[d]imidazol-2-yl)quinoxali...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610836 (MSC-4381) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610833 (CHEMBL5276884) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508530 ((R)-N-(2-(3-(4-cyano-3,5-difluorophenyl)-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107747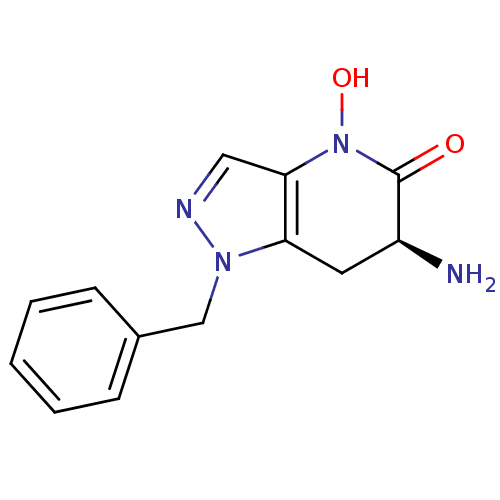 (CHEMBL2347115 | US8933095, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309988 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309988 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309990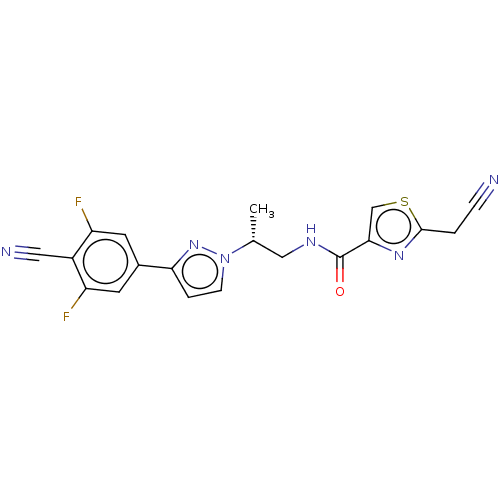 ((R)-N-(2-(3-(4-cyano-3,5-difluorophenyl)-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225573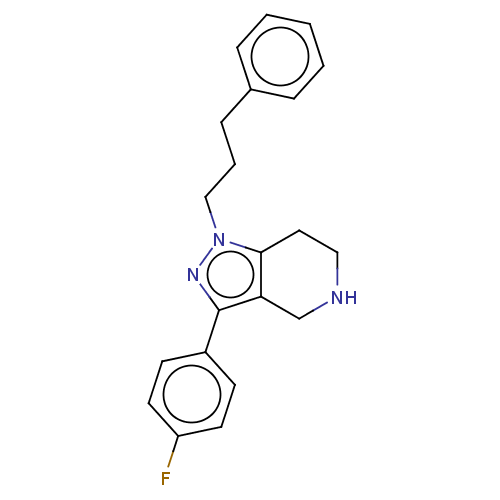 (CHEMBL173140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225671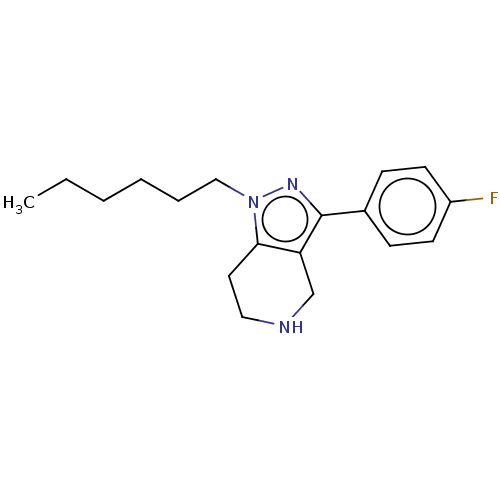 (CHEMBL369407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508521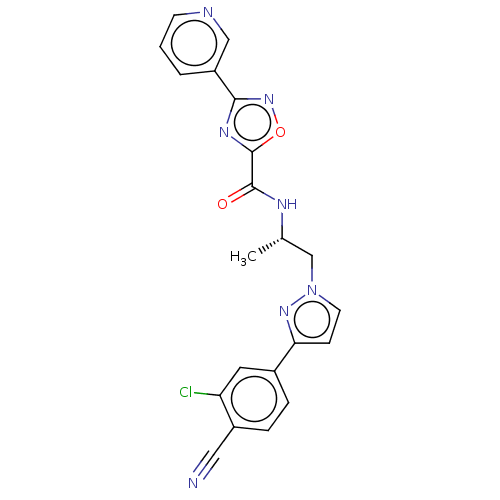 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309981 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309979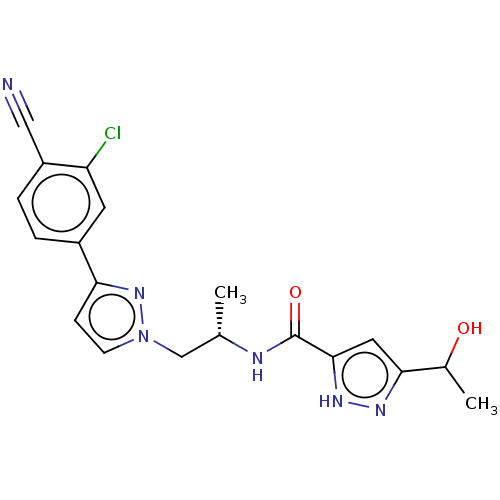 (N-((S)-1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508519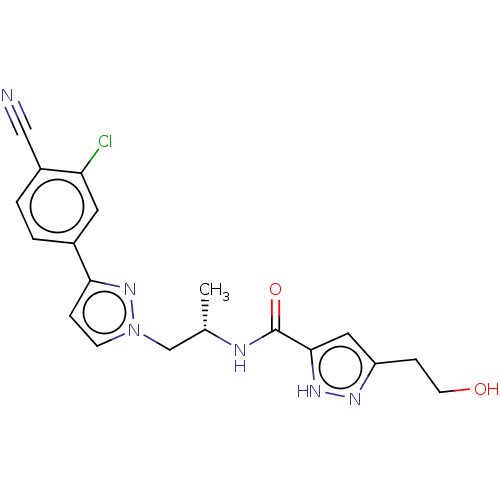 (N-((S)-1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309995 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292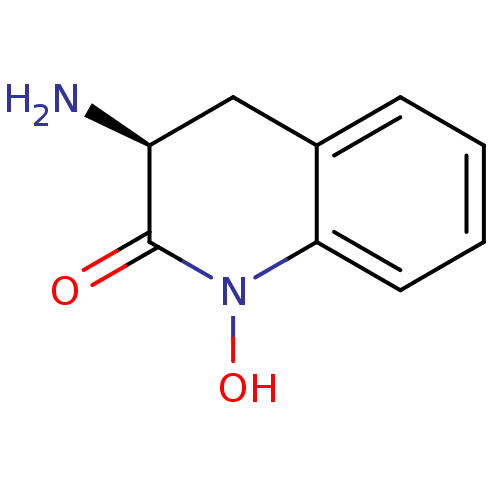 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508534 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225570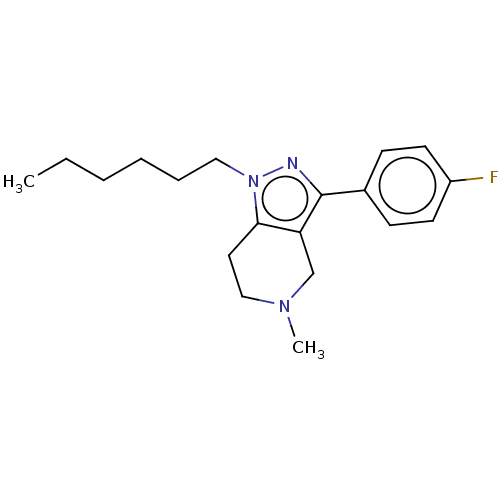 (CHEMBL171362) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225576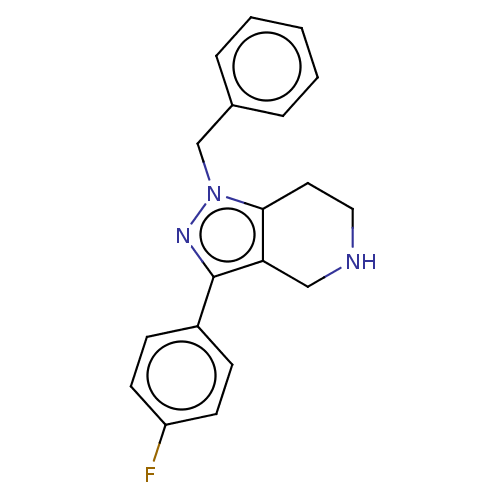 (CHEMBL535357) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from postsynaptic alpha-1 adrenergic receptor of rat in vitro. | J Med Chem 28: 934-40 (1985) BindingDB Entry DOI: 10.7270/Q29C70NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309976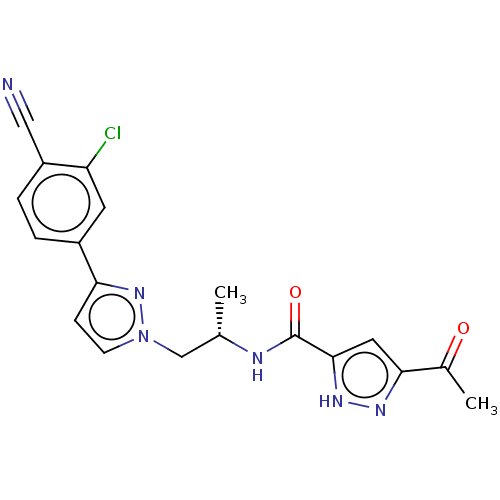 ((S)-3-acetyl-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309992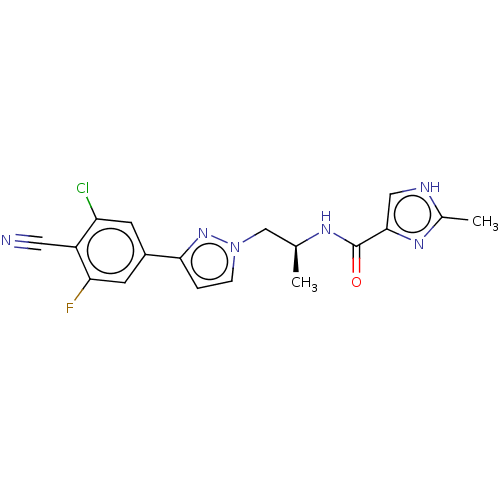 ((S)-N-{1-[3-(3-Chloro-4-cyano-5-fluorophenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cells | J Med Chem 48: 8253-60 (2005) Article DOI: 10.1021/jm050792d BindingDB Entry DOI: 10.7270/Q2RF5VTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610834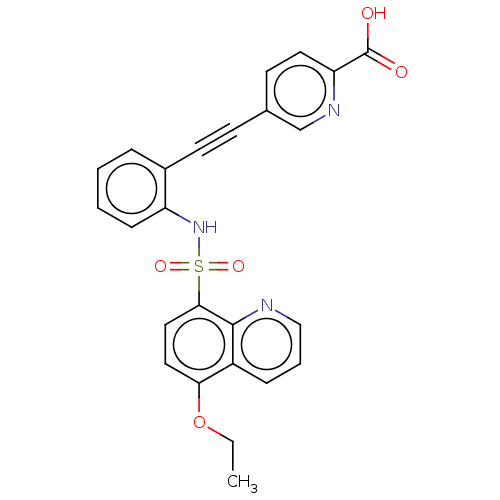 (CHEMBL5279064) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309976 ((S)-3-acetyl-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM309992 ((S)-N-{1-[3-(3-Chloro-4-cyano-5-fluorophenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | US Patent US9657003 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610835 (CHEMBL5267349) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM508523 ((S)-N-(1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Androgen receptor (AR) binding affinities of test compounds were studied in cytosolic lysates obtained from ventral prostates of castrated rats by co... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610830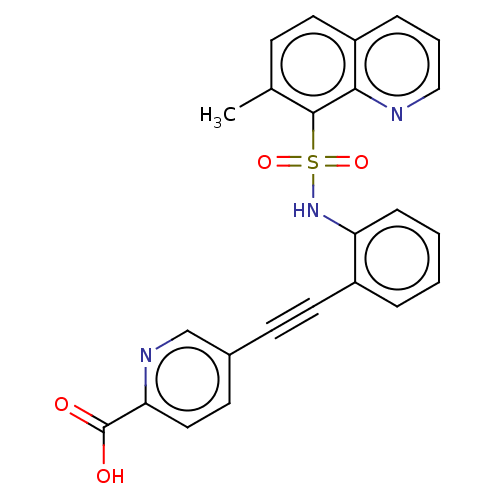 (CHEMBL5267752) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 4 (Homo sapiens (Human)) | BDBM50610837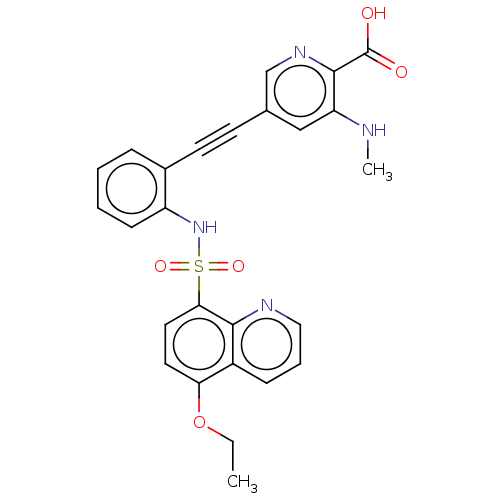 (CHEMBL5281492) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 717 total ) | Next | Last >> |