 Found 349 hits with Last Name = 'eatherton' and Initial = 'aj'
Found 349 hits with Last Name = 'eatherton' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP3 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50290085
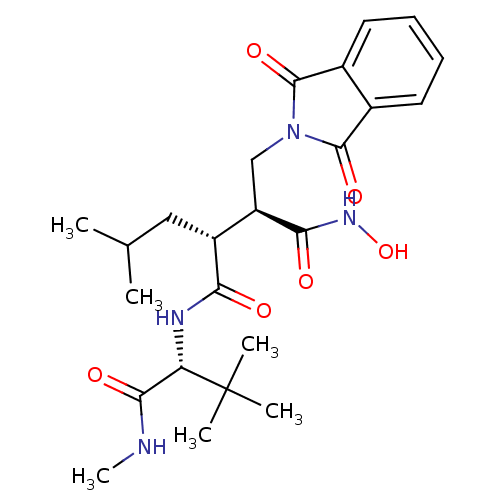
((2S,3R)-N*4*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](CN1C(=O)c2ccccc2C1=O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C24H34N4O6/c1-13(2)11-16(19(29)26-18(21(31)25-6)24(3,4)5)17(20(30)27-34)12-28-22(32)14-9-7-8-10-15(14)23(28)33/h7-10,13,16-18,34H,11-12H2,1-6H3,(H,25,31)(H,26,29)(H,27,30)/t16-,17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593694
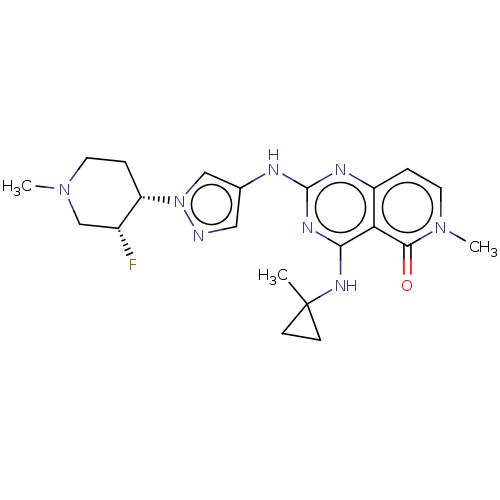
(CHEMBL5193253)Show SMILES CN1CC[C@@H]([C@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593696
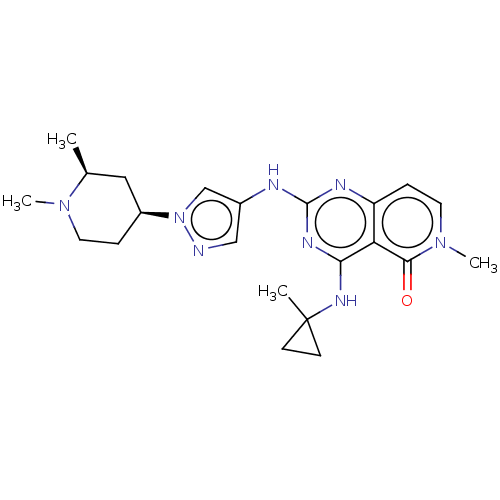
(CHEMBL5200601)Show SMILES C[C@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593693

(CHEMBL5201376)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593700

(CHEMBL5193024)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593697

(CHEMBL5196755)Show SMILES C[C@@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593695
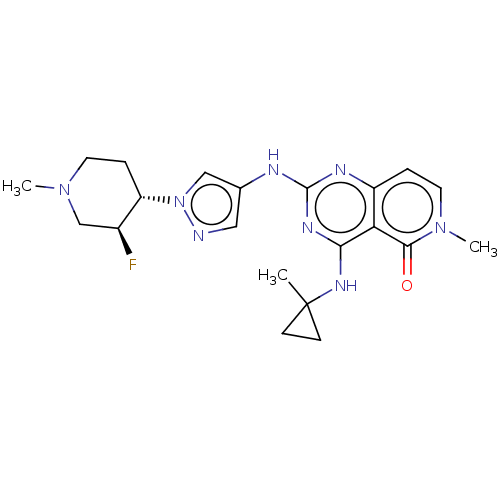
(CHEMBL5209502)Show SMILES CN1CC[C@@H]([C@@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593710
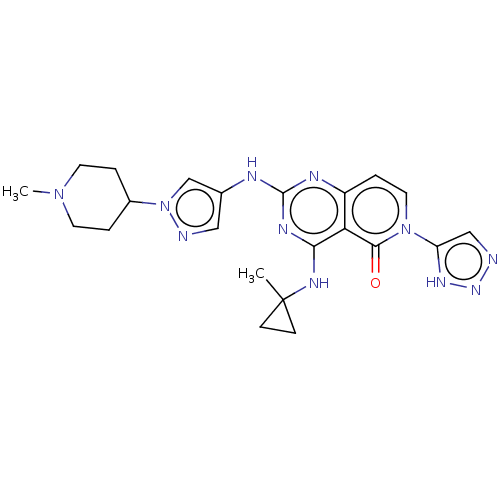
(CHEMBL5193053)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cnn[nH]4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593692

(CHEMBL5200432)Show SMILES CN(C)CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593689
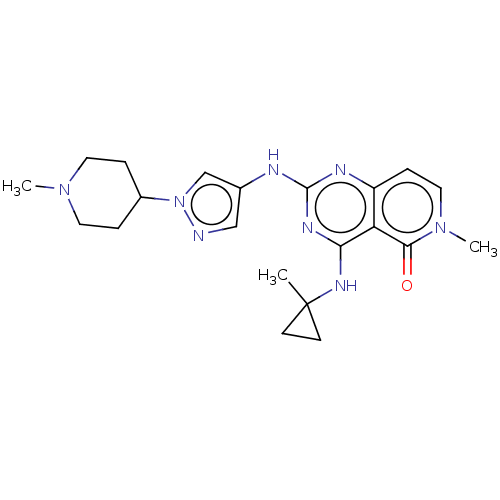
(CHEMBL5174529)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50290088

((2S,3R)-N*1*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](CN1C(=O)N(C)C(C)(C)C1=O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C22H39N5O6/c1-12(2)10-13(16(28)24-15(18(30)23-8)21(3,4)5)14(17(29)25-33)11-27-19(31)22(6,7)26(9)20(27)32/h12-15,33H,10-11H2,1-9H3,(H,23,30)(H,24,28)(H,25,29)/t13-,14-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593685
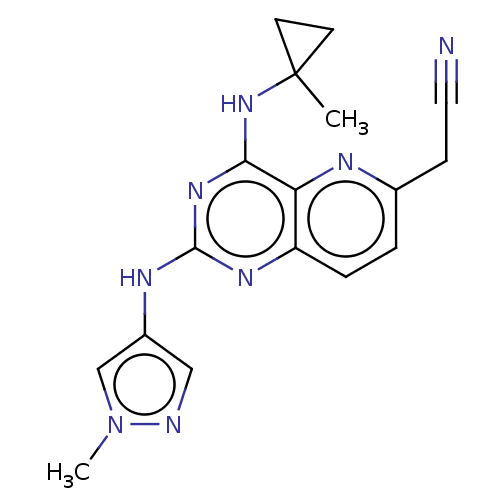
(CHEMBL5179237) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419410

(CHEMBL1915252)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H15ClN2O3/c1-12-7-17(20(24)25)22-23(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593691
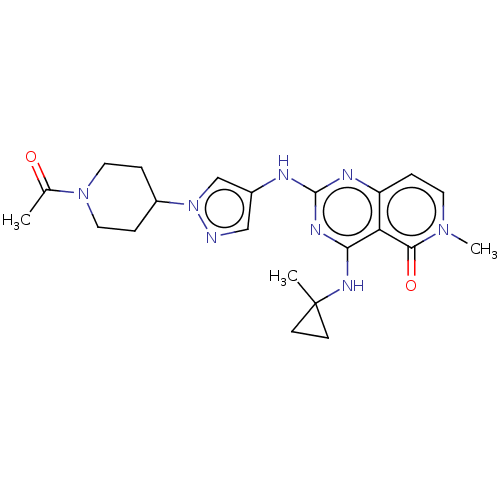
(CHEMBL5207332)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593690
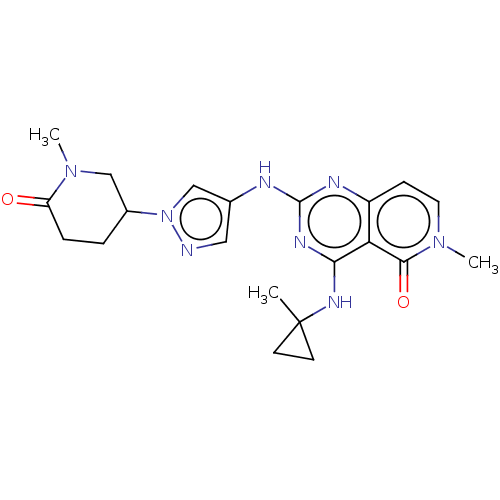
(CHEMBL5178970)Show SMILES CN1CC(CCC1=O)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593704
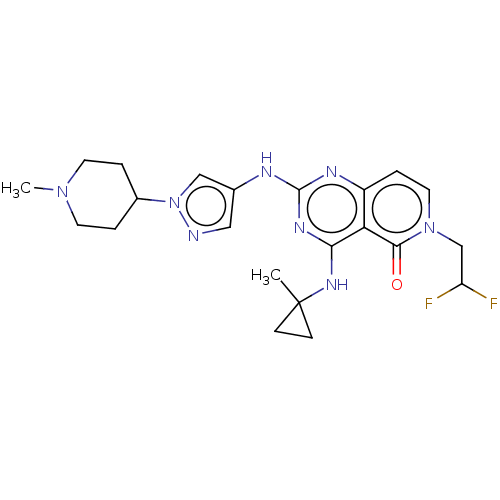
(CHEMBL5186874)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(CC(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593716

(CHEMBL5207654)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CC4(C3)CN(C)C4)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593706

(CHEMBL5190750)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50290089

((2R,3R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](C)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-9(2)8-11(10(3)13(20)19-23)14(21)18-12(15(22)17-7)16(4,5)6/h9-12,23H,8H2,1-7H3,(H,17,22)(H,18,21)(H,19,20)/t10-,11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human gelatinase B, MMP9 |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50290089

((2R,3R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](C)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-9(2)8-11(10(3)13(20)19-23)14(21)18-12(15(22)17-7)16(4,5)6/h9-12,23H,8H2,1-7H3,(H,17,22)(H,18,21)(H,19,20)/t10-,11-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50290088

((2S,3R)-N*1*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](CN1C(=O)N(C)C(C)(C)C1=O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C22H39N5O6/c1-12(2)10-13(16(28)24-15(18(30)23-8)21(3,4)5)14(17(29)25-33)11-27-19(31)22(6,7)26(9)20(27)32/h12-15,33H,10-11H2,1-9H3,(H,23,30)(H,24,28)(H,25,29)/t13-,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human gelatinase B, MMP9 |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593703
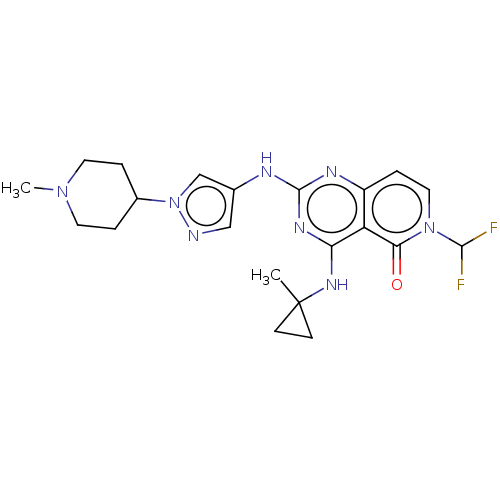
(CHEMBL5189582)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593698
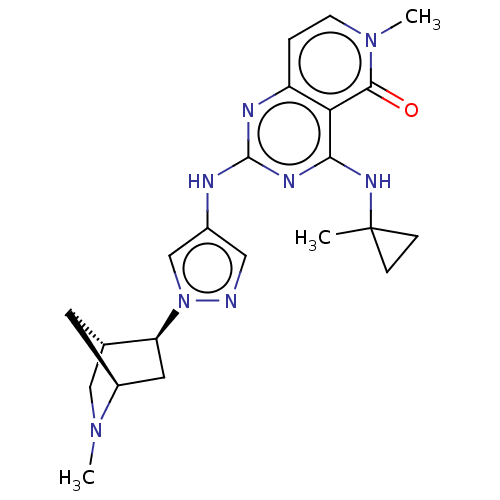
(CHEMBL5187604)Show SMILES [H][C@]12CN(C)[C@]([H])(C[C@@H]1n1cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn1)C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593715

(CHEMBL5177080)Show SMILES [H][C@@]12CN(C)[C@@]([H])(C[C@@H]1n1cc(Nc3nc(NC4(C)CC4)c4c(ccn(C[C@H](C)F)c4=O)n3)cn1)C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419415
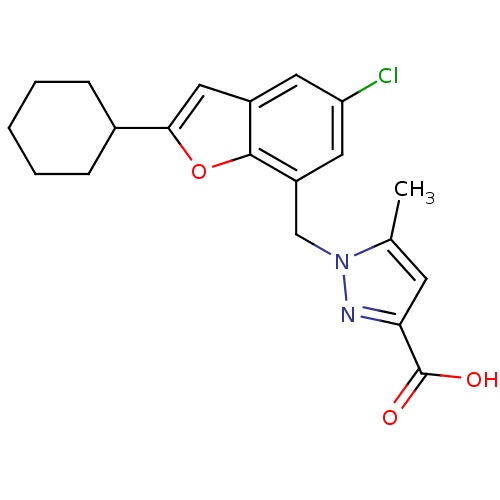
(CHEMBL1915015)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)C1CCCCC1)C(O)=O Show InChI InChI=1S/C20H21ClN2O3/c1-12-7-17(20(24)25)22-23(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h7-10,13H,2-6,11H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593713
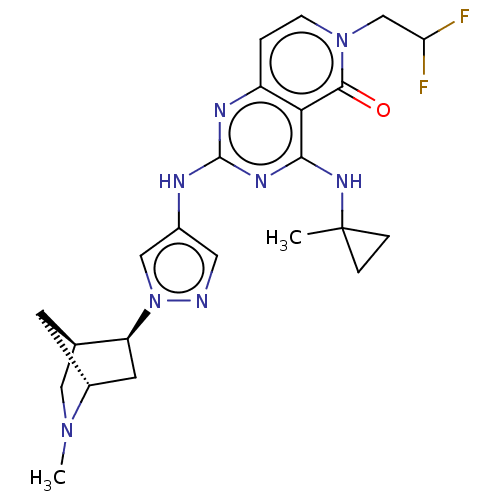
(CHEMBL5184407)Show SMILES [H][C@@]12CN(C)[C@@]([H])(C[C@@H]1n1cc(Nc3nc(NC4(C)CC4)c4c(ccn(CC(F)F)c4=O)n3)cn1)C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593714
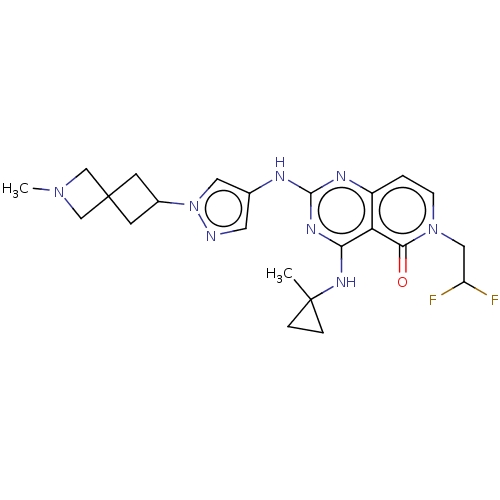
(CHEMBL5178692)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(CC(F)F)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593702
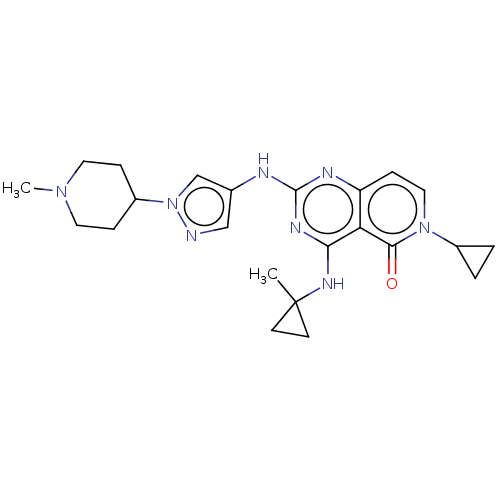
(CHEMBL5193495)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C4CC4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593709
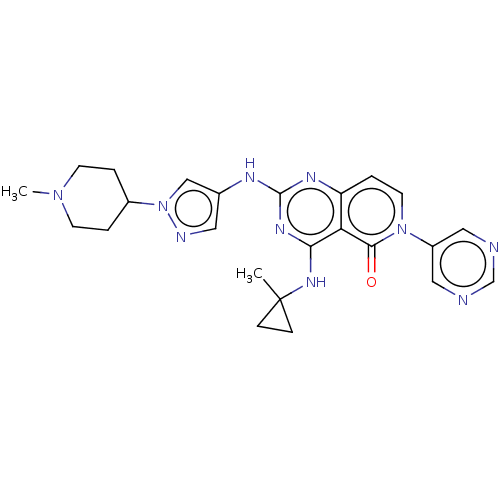
(CHEMBL5191035)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cncnc4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593708

(CHEMBL5188816)Show SMILES C[C@@H](CF)n1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50290085

((2S,3R)-N*4*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](CN1C(=O)c2ccccc2C1=O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C24H34N4O6/c1-13(2)11-16(19(29)26-18(21(31)25-6)24(3,4)5)17(20(30)27-34)12-28-22(32)14-9-7-8-10-15(14)23(28)33/h7-10,13,16-18,34H,11-12H2,1-6H3,(H,25,31)(H,26,29)(H,27,30)/t16-,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human gelatinase B, MMP9 |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50290086
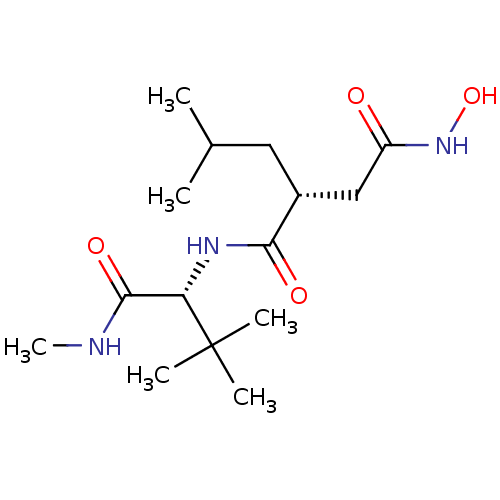
((R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)-pro...)Show SMILES CNC(=O)[C@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50505752

(CHEMBL4521212)Show SMILES COC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cnn(C)c2)nc2ccc(C)nc12 |r,wU:13.17,wD:10.10,(69.97,-6.35,;69.98,-4.81,;68.66,-4.03,;68.68,-2.49,;67.32,-4.78,;67.31,-6.33,;65.97,-7.08,;64.64,-6.3,;64.65,-4.76,;65.99,-4,;63.3,-7.06,;61.96,-6.28,;60.62,-7.05,;60.62,-8.58,;61.96,-9.37,;63.29,-8.6,;59.29,-9.36,;59.28,-10.9,;60.62,-11.68,;60.62,-13.22,;61.95,-13.99,;63.28,-13.22,;63.43,-11.69,;64.94,-11.37,;65.7,-12.7,;67.24,-12.7,;64.68,-13.84,;59.28,-13.99,;57.95,-13.22,;56.63,-14,;55.29,-13.25,;55.27,-11.7,;53.93,-10.95,;56.6,-10.92,;57.94,-11.68,)| Show InChI InChI=1S/C24H33N9O2/c1-16-4-9-20-21(26-16)22(30-23(29-20)28-18-14-25-31(2)15-18)27-17-5-7-19(8-6-17)32-10-12-33(13-11-32)24(34)35-3/h4,9,14-15,17,19H,5-8,10-13H2,1-3H3,(H2,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593686

(CHEMBL5173450)Show SMILES COC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cnn(C)c2)nc2ccn(C)c(=O)c12 |r,wU:13.17,wD:10.10,(5.33,6.16,;6.67,5.39,;6.67,3.85,;8,3.08,;5.33,3.08,;4,3.85,;2.67,3.08,;2.67,1.54,;4,.77,;5.33,1.54,;1.33,.77,;-0,1.54,;-1.33,.77,;-1.33,-.77,;-0,-1.54,;1.33,-.77,;-2.67,-1.54,;-2.67,-3.08,;-1.33,-3.85,;-1.33,-5.38,;0,-6.15,;1.34,-5.38,;1.34,-3.84,;2.84,-3.38,;3.72,-4.6,;5.26,-4.6,;2.8,-5.84,;-2.66,-6.16,;-4,-5.39,;-5.33,-6.16,;-6.67,-5.39,;-6.67,-3.85,;-8,-3.08,;-5.33,-3.08,;-5.33,-1.54,;-4,-3.85,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593707
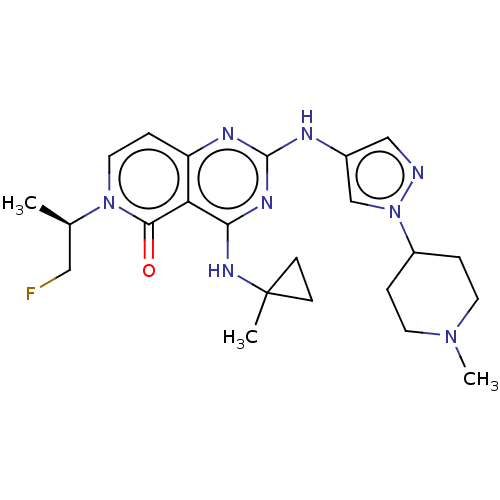
(CHEMBL5193438)Show SMILES C[C@H](CF)n1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593701
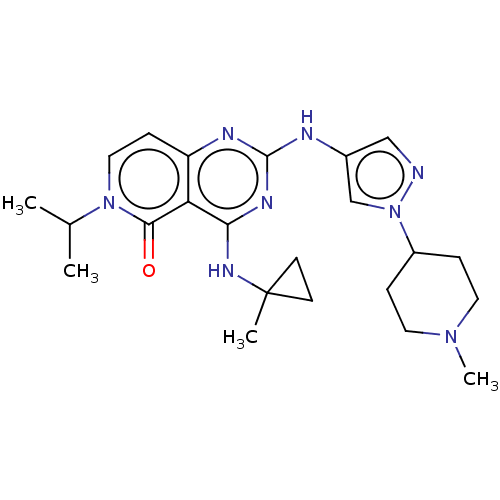
(CHEMBL5205770)Show SMILES CC(C)n1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419403
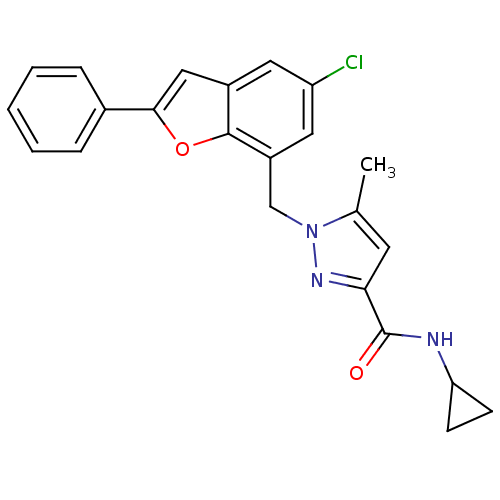
(CHEMBL1914467)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NC1CC1 Show InChI InChI=1S/C23H20ClN3O2/c1-14-9-20(23(28)25-19-7-8-19)26-27(14)13-17-11-18(24)10-16-12-21(29-22(16)17)15-5-3-2-4-6-15/h2-6,9-12,19H,7-8,13H2,1H3,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419417
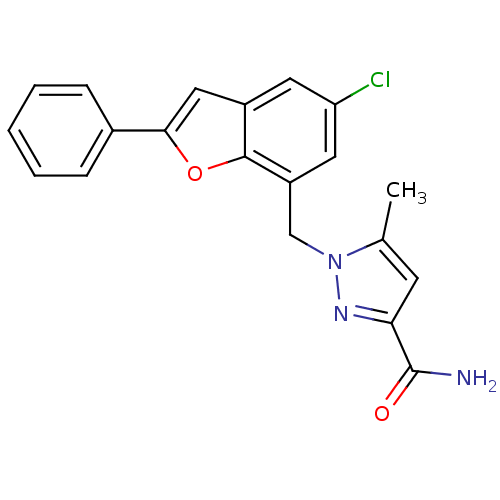
(CHEMBL1915254)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(N)=O Show InChI InChI=1S/C20H16ClN3O2/c1-12-7-17(20(22)25)23-24(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H2,22,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP1 receptor expressed in CHO-K1 cells after 30 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
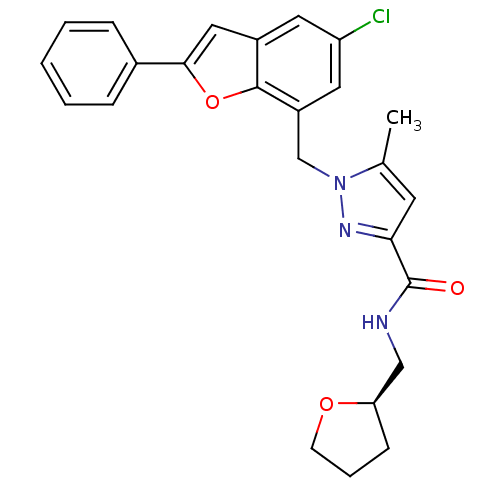
(Homo sapiens (Human)) | BDBM50593711
(CHEMBL5190559)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cn(C)nn4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419419
(CHEMBL1915262)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NC[C@H]1CCCO1 |r| Show InChI InChI=1S/C25H24ClN3O3/c1-16-10-22(25(30)27-14-21-8-5-9-31-21)28-29(16)15-19-12-20(26)11-18-13-23(32-24(18)19)17-6-3-2-4-7-17/h2-4,6-7,10-13,21H,5,8-9,14-15H2,1H3,(H,27,30)/t21-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50290090
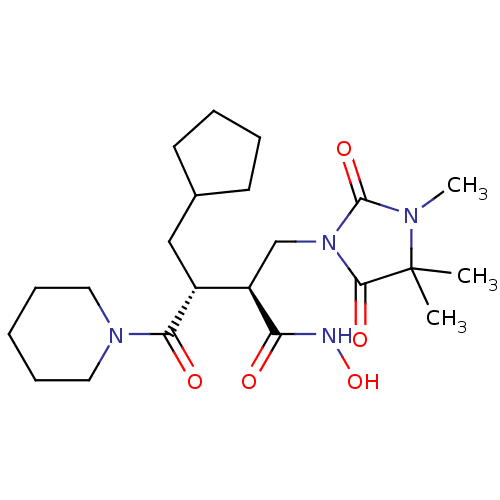
((2S,3R)-3-Cyclopentylmethyl-N-hydroxy-4-oxo-4-pipe...)Show SMILES CN1C(=O)N(C[C@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2299-2302 (1997)
Article DOI: 10.1016/S0960-894X(97)00416-2
BindingDB Entry DOI: 10.7270/Q26T0MM8 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50269801

(CHEMBL4100294)Show InChI InChI=1S/C19H14BrFN2O2/c20-14-5-8-18(25-12-13-3-6-15(21)7-4-13)17(10-14)19(24)23-16-2-1-9-22-11-16/h1-11H,12H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurodegeneration DPU, Neurosciences Therapeutic Area Unit, GSK Pharmaceuticals R&D, 898 Halei Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai 201203, PR China.
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 27: 4034-4038 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.052
BindingDB Entry DOI: 10.7270/Q2MG7S1N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50269809
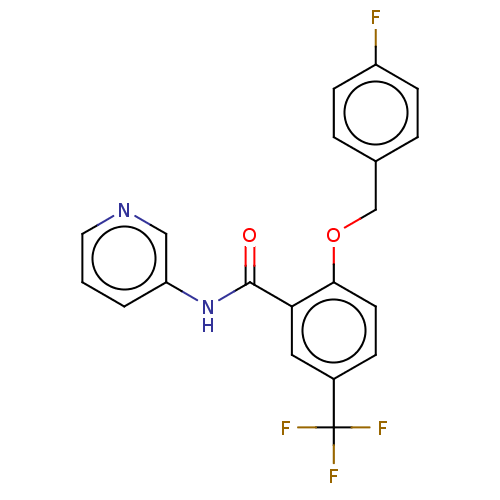
(CHEMBL4077195)Show SMILES Fc1ccc(COc2ccc(cc2C(=O)Nc2cccnc2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H14F4N2O2/c21-15-6-3-13(4-7-15)12-28-18-8-5-14(20(22,23)24)10-17(18)19(27)26-16-2-1-9-25-11-16/h1-11H,12H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurodegeneration DPU, Neurosciences Therapeutic Area Unit, GSK Pharmaceuticals R&D, 898 Halei Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai 201203, PR China.
Curated by ChEMBL
| Assay Description
Inhibition of human LRRK2 using RLGRDKYKTLRQIRQ as substrate in presence of [gamma-33P]-ATP |
Bioorg Med Chem Lett 27: 4034-4038 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.052
BindingDB Entry DOI: 10.7270/Q2MG7S1N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50269824
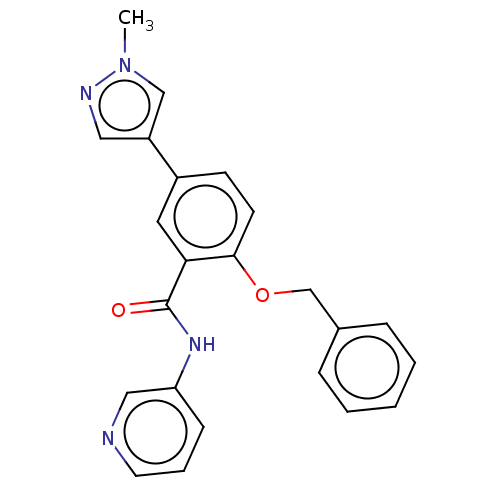
(CHEMBL4098650)Show SMILES Cn1cc(cn1)-c1ccc(OCc2ccccc2)c(c1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C23H20N4O2/c1-27-15-19(13-25-27)18-9-10-22(29-16-17-6-3-2-4-7-17)21(12-18)23(28)26-20-8-5-11-24-14-20/h2-15H,16H2,1H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurodegeneration DPU, Neurosciences Therapeutic Area Unit, GSK Pharmaceuticals R&D, 898 Halei Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai 201203, PR China.
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 27: 4034-4038 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.052
BindingDB Entry DOI: 10.7270/Q2MG7S1N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50269800
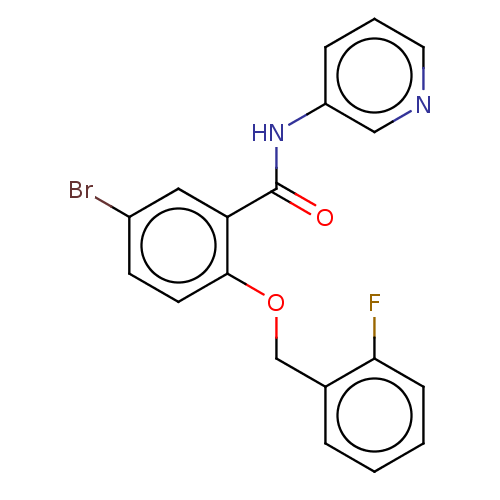
(CHEMBL4068065)Show InChI InChI=1S/C19H14BrFN2O2/c20-14-7-8-18(25-12-13-4-1-2-6-17(13)21)16(10-14)19(24)23-15-5-3-9-22-11-15/h1-11H,12H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurodegeneration DPU, Neurosciences Therapeutic Area Unit, GSK Pharmaceuticals R&D, 898 Halei Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai 201203, PR China.
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 27: 4034-4038 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.052
BindingDB Entry DOI: 10.7270/Q2MG7S1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419408
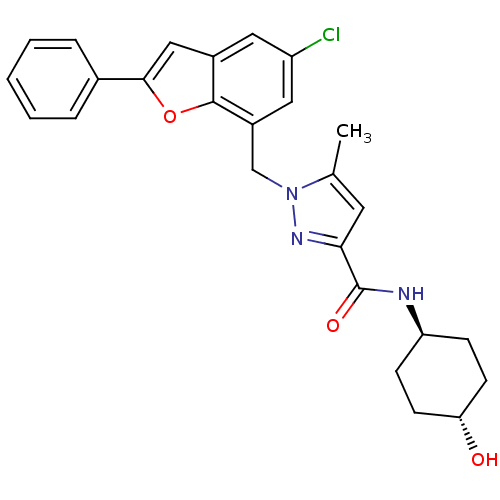
(CHEMBL1915261)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:26.29,wD:29.33,(19.51,-3.15,;19.53,-1.54,;21.04,-1.85,;21.81,-.51,;20.77,.63,;19.36,-.01,;18.02,.76,;16.69,-.02,;15.36,.75,;14.03,-.02,;12.69,.75,;14.03,-1.57,;15.36,-2.34,;15.68,-3.85,;17.21,-4.01,;17.84,-2.6,;16.7,-1.57,;17.98,-5.35,;17.2,-6.67,;17.96,-8.01,;19.51,-8.01,;20.28,-6.67,;19.51,-5.34,;23.34,-.35,;23.96,1.06,;24.25,-1.59,;25.78,-1.59,;26.55,-.26,;28.09,-.26,;28.85,-1.59,;30.39,-1.6,;28.08,-2.92,;26.55,-2.92,)| Show InChI InChI=1S/C26H26ClN3O3/c1-16-11-23(26(32)28-21-7-9-22(31)10-8-21)29-30(16)15-19-13-20(27)12-18-14-24(33-25(18)19)17-5-3-2-4-6-17/h2-6,11-14,21-22,31H,7-10,15H2,1H3,(H,28,32)/t21-,22- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593688
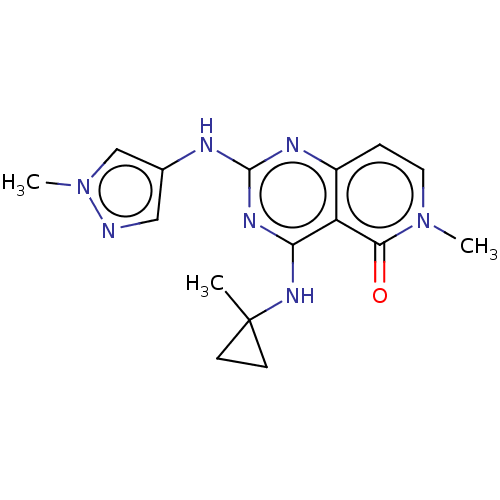
(CHEMBL5183067)Show SMILES Cn1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data