 Found 106 hits with Last Name = 'taylor' and Initial = 'aj'
Found 106 hits with Last Name = 'taylor' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160371
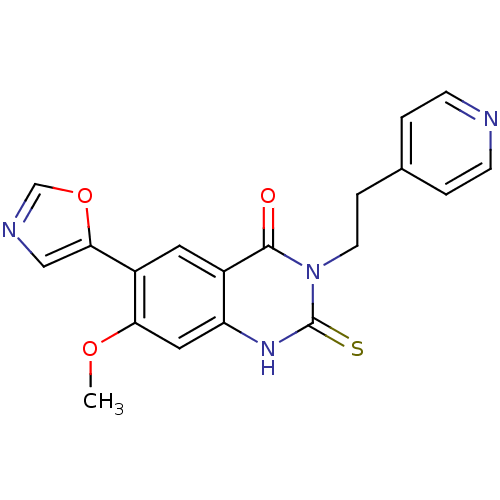
(7-Methoxy-6-oxazol-5-yl-3-(2-pyridin-4-yl-ethyl)-2...)Show SMILES COc1cc2[nH]c(=S)n(CCc3ccncc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C19H16N4O3S/c1-25-16-9-15-13(8-14(16)17-10-21-11-26-17)18(24)23(19(27)22-15)7-4-12-2-5-20-6-3-12/h2-3,5-6,8-11H,4,7H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160379
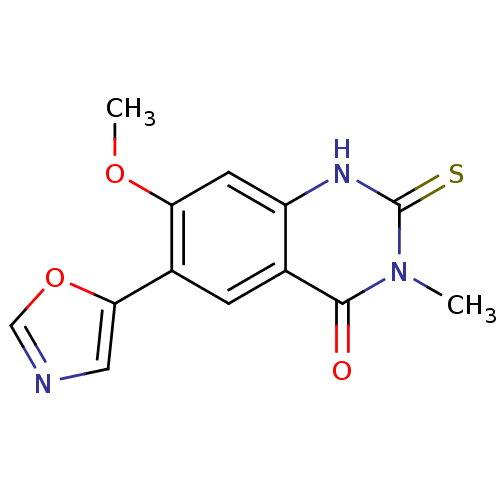
(7-Methoxy-3-methyl-6-oxazol-5-yl-2-thioxo-2,3-dihy...)Show InChI InChI=1S/C13H11N3O3S/c1-16-12(17)7-3-8(11-5-14-6-19-11)10(18-2)4-9(7)15-13(16)20/h3-6H,1-2H3,(H,15,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160378

(3-(3-Imidazol-1-yl-propyl)-7-methoxy-6-oxazol-5-yl...)Show SMILES COc1cc2[nH]c(=S)n(CCCn3ccnc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C18H17N5O3S/c1-25-15-8-14-12(7-13(15)16-9-20-11-26-16)17(24)23(18(27)21-14)5-2-4-22-6-3-19-10-22/h3,6-11H,2,4-5H2,1H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160375
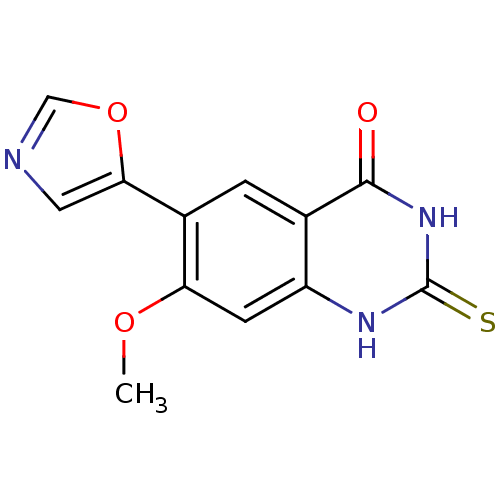
(7-Methoxy-6-oxazol-5-yl-2-thioxo-2,3-dihydro-1H-qu...)Show InChI InChI=1S/C12H9N3O3S/c1-17-9-3-8-6(11(16)15-12(19)14-8)2-7(9)10-4-13-5-18-10/h2-5H,1H3,(H2,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174783
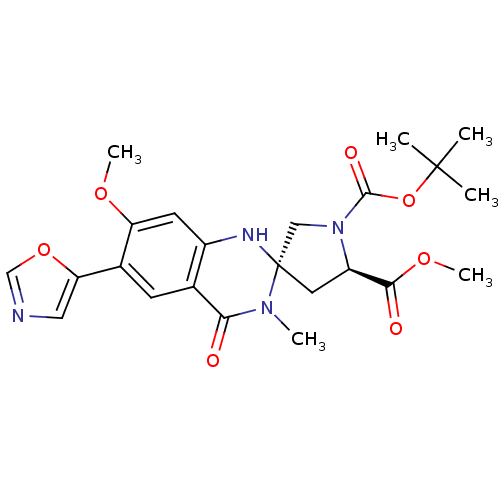
(1-tert-butyl 5-methyl (3S,5R)-7'-methoxy-3'-methyl...)Show SMILES COC(=O)[C@H]1C[C@]2(CN1C(=O)OC(C)(C)C)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C23H28N4O7/c1-22(2,3)34-21(30)27-11-23(9-16(27)20(29)32-6)25-15-8-17(31-5)14(18-10-24-12-33-18)7-13(15)19(28)26(23)4/h7-8,10,12,16,25H,9,11H2,1-6H3/t16-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174782

(CHEMBL198729 | N,N-diethyl-7'-methoxy-3'-methyl-6'...)Show SMILES CCN(CC)C(=O)N1CCC2(C1)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C21H27N5O4/c1-5-25(6-2)20(28)26-8-7-21(12-26)23-16-10-17(29-4)15(18-11-22-13-30-18)9-14(16)19(27)24(21)3/h9-11,13,23H,5-8,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160374
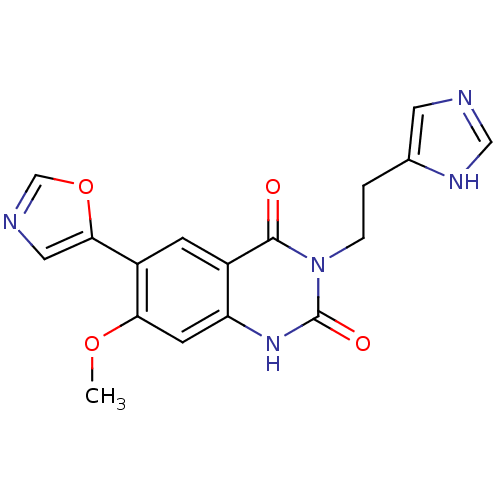
(3-[2-(1H-Imidazol-4-yl)-ethyl]-7-methoxy-6-oxazol-...)Show SMILES COc1cc2[nH]c(=O)n(CCc3cnc[nH]3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C17H15N5O4/c1-25-14-5-13-11(4-12(14)15-7-19-9-26-15)16(23)22(17(24)21-13)3-2-10-6-18-8-20-10/h4-9H,2-3H2,1H3,(H,18,20)(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174790

(7-methoxy-2,3-dimethyl-6-(oxazol-5-yl)-2-styryl-2,...)Show SMILES COc1cc2NC(C)(\C=C\c3ccccc3)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C22H21N3O3/c1-22(10-9-15-7-5-4-6-8-15)24-18-12-19(27-3)17(20-13-23-14-28-20)11-16(18)21(26)25(22)2/h4-14,24H,1-3H3/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174792
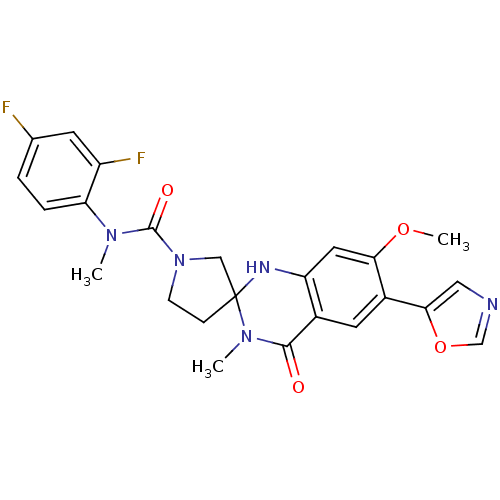
(CHEMBL199075 | N-(2,4-difluorophenyl)-7'-methoxy-N...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)N(C)c3ccc(F)cc3F)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C24H23F2N5O4/c1-29(19-5-4-14(25)8-17(19)26)23(33)31-7-6-24(12-31)28-18-10-20(34-3)16(21-11-27-13-35-21)9-15(18)22(32)30(24)2/h4-5,8-11,13,28H,6-7,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174796
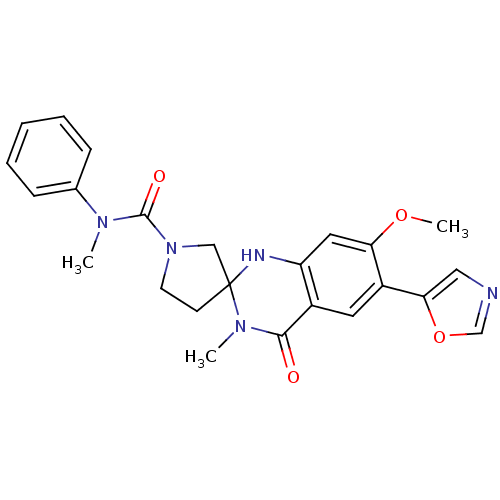
(7'-methoxy-N,3'-dimethyl-6'-(1,3-oxazol-5-yl)-4'-o...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)N(C)c3ccccc3)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C24H25N5O4/c1-27(16-7-5-4-6-8-16)23(31)29-10-9-24(14-29)26-19-12-20(32-3)18(21-13-25-15-33-21)11-17(19)22(30)28(24)2/h4-8,11-13,15,26H,9-10,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174795
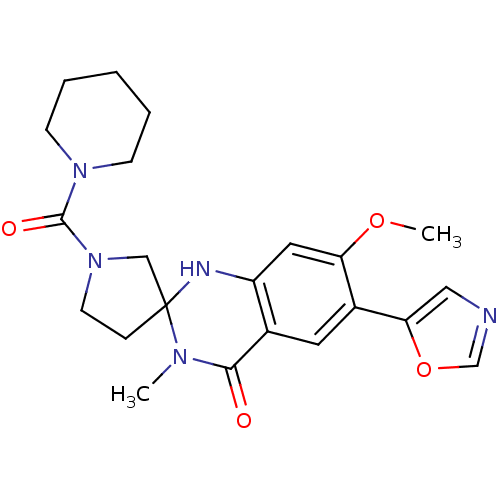
(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-1-[(pipe...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)N3CCCCC3)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C22H27N5O4/c1-25-20(28)15-10-16(19-12-23-14-31-19)18(30-2)11-17(15)24-22(25)6-9-27(13-22)21(29)26-7-4-3-5-8-26/h10-12,14,24H,3-9,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174789
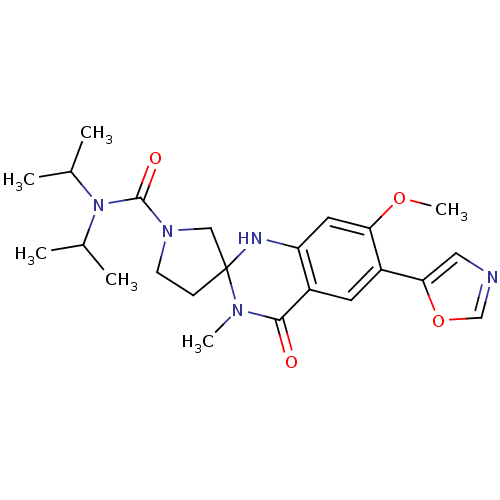
(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-4'-oxo-N...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)N(C(C)C)C(C)C)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C23H31N5O4/c1-14(2)28(15(3)4)22(30)27-8-7-23(12-27)25-18-10-19(31-6)17(20-11-24-13-32-20)9-16(18)21(29)26(23)5/h9-11,13-15,25H,7-8,12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160368
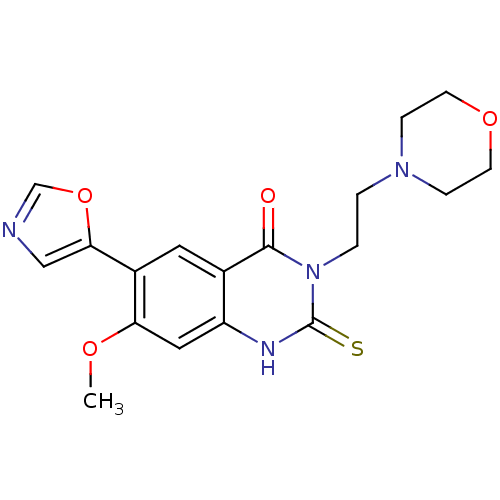
(7-Methoxy-3-(2-morpholin-4-yl-ethyl)-6-oxazol-5-yl...)Show SMILES COc1cc2[nH]c(=S)n(CCN3CCOCC3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C18H20N4O4S/c1-24-15-9-14-12(8-13(15)16-10-19-11-26-16)17(23)22(18(27)20-14)3-2-21-4-6-25-7-5-21/h8-11H,2-7H2,1H3,(H,20,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174785
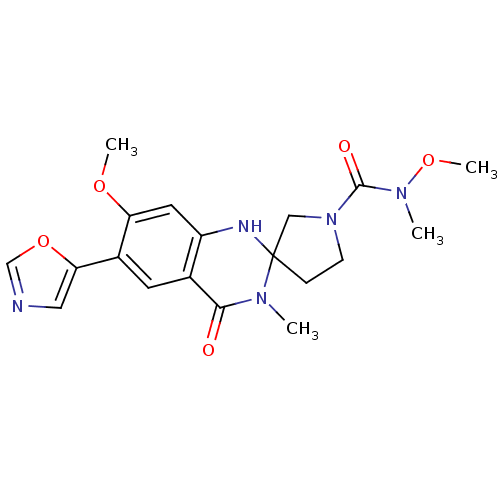
(CHEMBL372753 | N,7'-dimethoxy-N,3'-dimethyl-6'-(1,...)Show SMILES CON(C)C(=O)N1CCC2(C1)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C19H23N5O5/c1-22-17(25)12-7-13(16-9-20-11-29-16)15(27-3)8-14(12)21-19(22)5-6-24(10-19)18(26)23(2)28-4/h7-9,11,21H,5-6,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174791
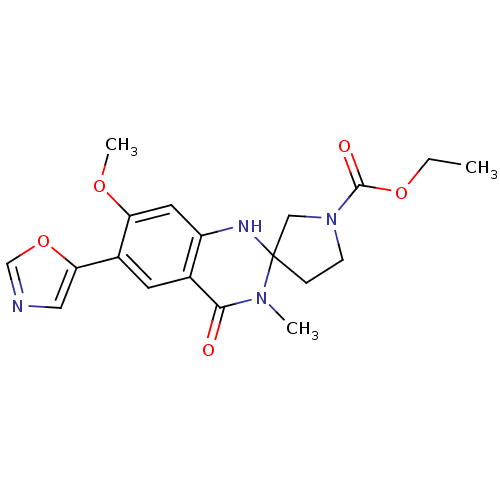
(CHEMBL197163 | ethyl 7'-methoxy-3'-methyl-6'-(1,3-...)Show SMILES CCOC(=O)N1CCC2(C1)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C19H22N4O5/c1-4-27-18(25)23-6-5-19(10-23)21-14-8-15(26-3)13(16-9-20-11-28-16)7-12(14)17(24)22(19)2/h7-9,11,21H,4-6,10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160370
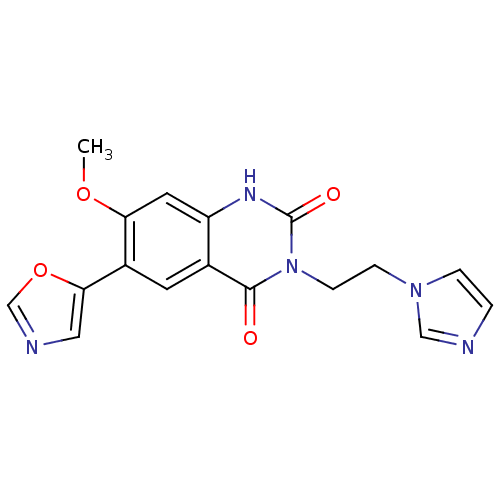
(3-(2-Imidazol-1-yl-ethyl)-7-methoxy-6-oxazol-5-yl-...)Show SMILES COc1cc2[nH]c(=O)n(CCn3ccnc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C17H15N5O4/c1-25-14-7-13-11(6-12(14)15-8-19-10-26-15)16(23)22(17(24)20-13)5-4-21-3-2-18-9-21/h2-3,6-10H,4-5H2,1H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160372
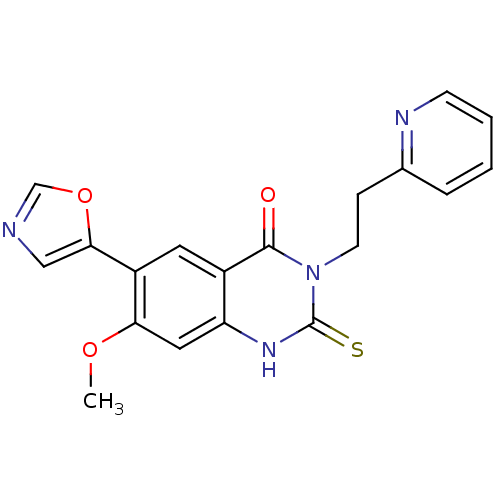
(7-Methoxy-6-oxazol-5-yl-3-(2-pyridin-2-yl-ethyl)-2...)Show SMILES COc1cc2[nH]c(=S)n(CCc3ccccn3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C19H16N4O3S/c1-25-16-9-15-13(8-14(16)17-10-20-11-26-17)18(24)23(19(27)22-15)7-5-12-4-2-3-6-21-12/h2-4,6,8-11H,5,7H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174788

(1-(2,2-dimethylpropanoyl)-7'-methoxy-3'-methyl-6'-...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)C(C)(C)C)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C21H26N4O4/c1-20(2,3)19(27)25-7-6-21(11-25)23-15-9-16(28-5)14(17-10-22-12-29-17)8-13(15)18(26)24(21)4/h8-10,12,23H,6-7,11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174781
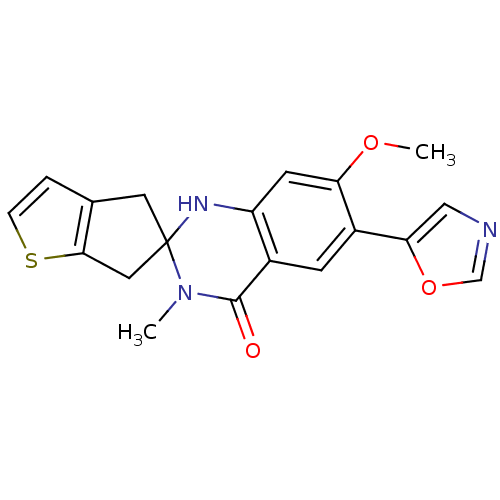
(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-3',4',1,...)Show SMILES COc1cc2NC3(Cc4ccsc4C3)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C19H17N3O3S/c1-22-18(23)12-5-13(16-9-20-10-25-16)15(24-2)6-14(12)21-19(22)7-11-3-4-26-17(11)8-19/h3-6,9-10,21H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160373
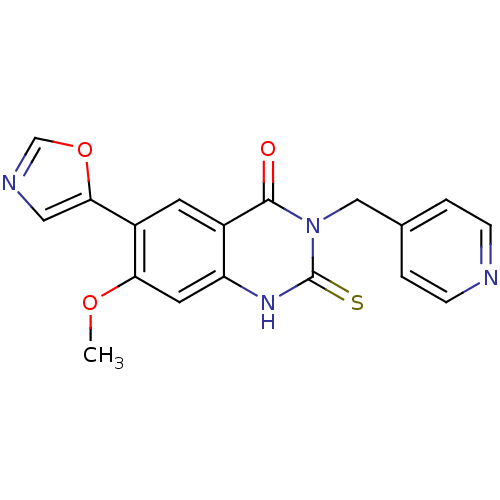
(7-Methoxy-6-oxazol-5-yl-3-pyridin-4-ylmethyl-2-thi...)Show SMILES COc1cc2[nH]c(=S)n(Cc3ccncc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C18H14N4O3S/c1-24-15-7-14-12(6-13(15)16-8-20-10-25-16)17(23)22(18(26)21-14)9-11-2-4-19-5-3-11/h2-8,10H,9H2,1H3,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160367
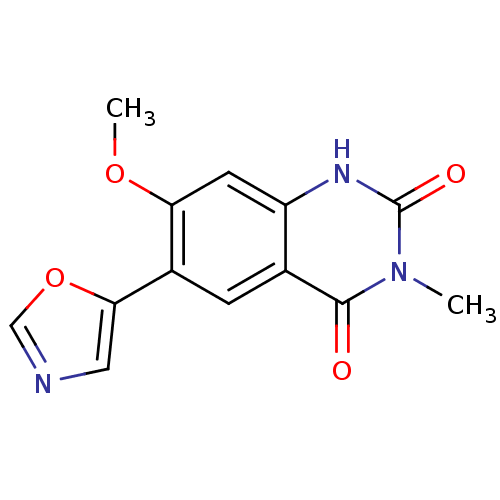
(7-Methoxy-3-methyl-6-oxazol-5-yl-1H-quinazoline-2,...)Show InChI InChI=1S/C13H11N3O4/c1-16-12(17)7-3-8(11-5-14-6-20-11)10(19-2)4-9(7)15-13(16)18/h3-6H,1-2H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160367

(7-Methoxy-3-methyl-6-oxazol-5-yl-1H-quinazoline-2,...)Show InChI InChI=1S/C13H11N3O4/c1-16-12(17)7-3-8(11-5-14-6-20-11)10(19-2)4-9(7)15-13(16)18/h3-6H,1-2H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174793

(CHEMBL371533 | tert-butyl 7'-methoxy-6'-(1,3-oxazo...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)OC(C)(C)C)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C21H26N4O5/c1-20(2,3)30-19(27)25-7-6-21(11-25)23-15-9-16(28-5)14(17-10-22-12-29-17)8-13(15)18(26)24(21)4/h8-10,12,23H,6-7,11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174798
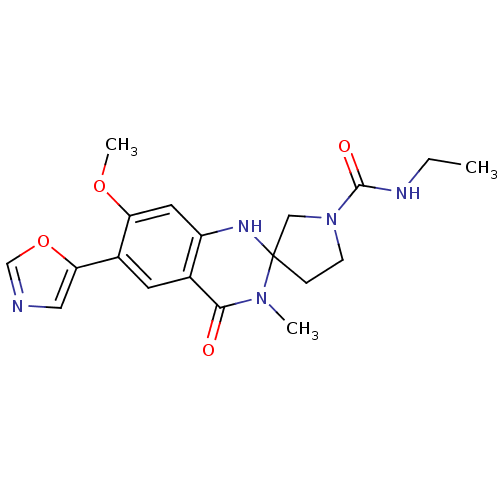
(CHEMBL373029 | N-ethyl-7'-methoxy-3'-methyl-6'-(1,...)Show SMILES CCNC(=O)N1CCC2(C1)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C19H23N5O4/c1-4-21-18(26)24-6-5-19(10-24)22-14-8-15(27-3)13(16-9-20-11-28-16)7-12(14)17(25)23(19)2/h7-9,11,22H,4-6,10H2,1-3H3,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160369
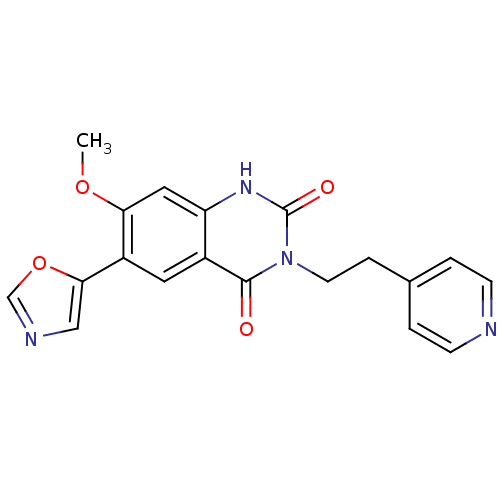
(7-Methoxy-6-oxazol-5-yl-3-(2-pyridin-4-yl-ethyl)-1...)Show SMILES COc1cc2[nH]c(=O)n(CCc3ccncc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C19H16N4O4/c1-26-16-9-15-13(8-14(16)17-10-21-11-27-17)18(24)23(19(25)22-15)7-4-12-2-5-20-6-3-12/h2-3,5-6,8-11H,4,7H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
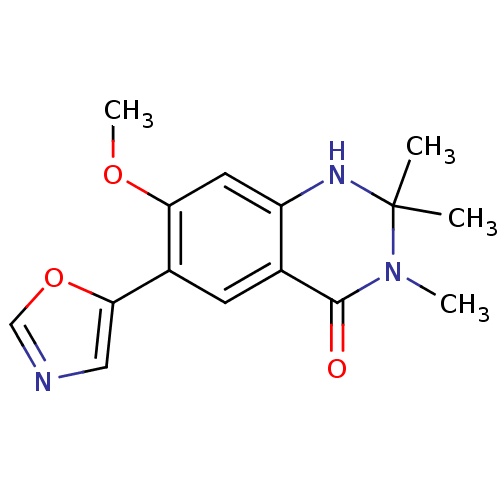
(Homo sapiens (Human)) | BDBM50174786
(7-methoxy-2,2,3-trimethyl-6-(oxazol-5-yl)-2,3-dihy...)Show InChI InChI=1S/C15H17N3O3/c1-15(2)17-11-6-12(20-4)10(13-7-16-8-21-13)5-9(11)14(19)18(15)3/h5-8,17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160380

(7-Methoxy-3-(2-morpholin-4-yl-ethyl)-6-oxazol-5-yl...)Show SMILES COc1cc2[nH]c(=O)n(CCN3CCOCC3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C18H20N4O5/c1-25-15-9-14-12(8-13(15)16-10-19-11-27-16)17(23)22(18(24)20-14)3-2-21-4-6-26-7-5-21/h8-11H,2-7H2,1H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174780
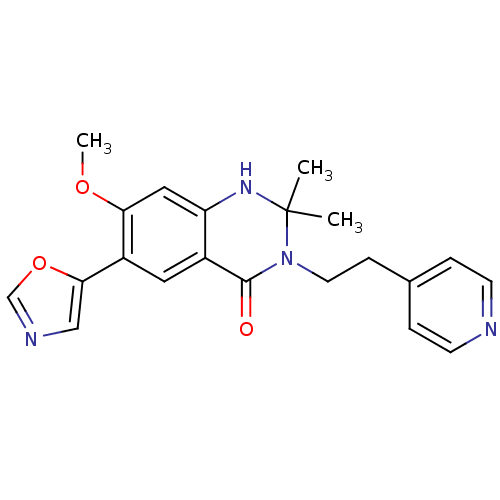
(7-methoxy-2,2-dimethyl-6-(oxazol-5-yl)-3-(2-(pyrid...)Show SMILES COc1cc2NC(C)(C)N(CCc3ccncc3)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C21H22N4O3/c1-21(2)24-17-11-18(27-3)16(19-12-23-13-28-19)10-15(17)20(26)25(21)9-6-14-4-7-22-8-5-14/h4-5,7-8,10-13,24H,6,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174784
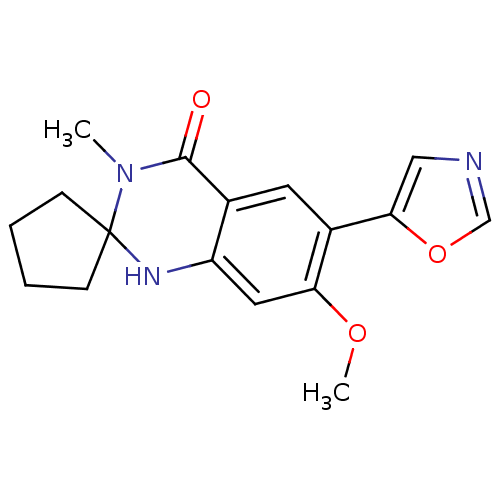
(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-1'H-spir...)Show InChI InChI=1S/C17H19N3O3/c1-20-16(21)11-7-12(15-9-18-10-23-15)14(22-2)8-13(11)19-17(20)5-3-4-6-17/h7-10,19H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174799
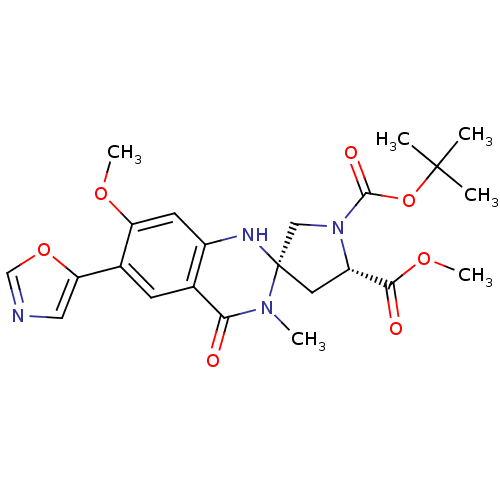
(1-tert-butyl 5-methyl (3S,5S)-7'-methoxy-3'-methyl...)Show SMILES COC(=O)[C@@H]1C[C@]2(CN1C(=O)OC(C)(C)C)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C23H28N4O7/c1-22(2,3)34-21(30)27-11-23(9-16(27)20(29)32-6)25-15-8-17(31-5)14(18-10-24-12-33-18)7-13(15)19(28)26(23)4/h7-8,10,12,16,25H,9,11H2,1-6H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174797

(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-4'-oxo-N...)Show SMILES COc1cc2NC3(CCN(C3)C(=O)Nc3ccccc3)N(C)C(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C23H23N5O4/c1-27-21(29)16-10-17(20-12-24-14-32-20)19(31-2)11-18(16)26-23(27)8-9-28(13-23)22(30)25-15-6-4-3-5-7-15/h3-7,10-12,14,26H,8-9,13H2,1-2H3,(H,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174787
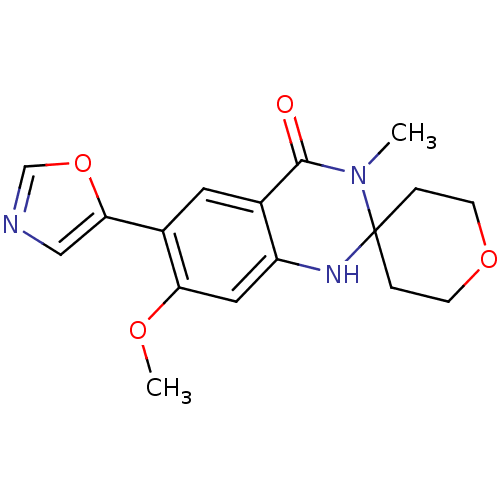
(7'-methoxy-3'-methyl-6'-(1,3-oxazol-5-yl)-2,3,5,6-...)Show InChI InChI=1S/C17H19N3O4/c1-20-16(21)11-7-12(15-9-18-10-24-15)14(22-2)8-13(11)19-17(20)3-5-23-6-4-17/h7-10,19H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160377
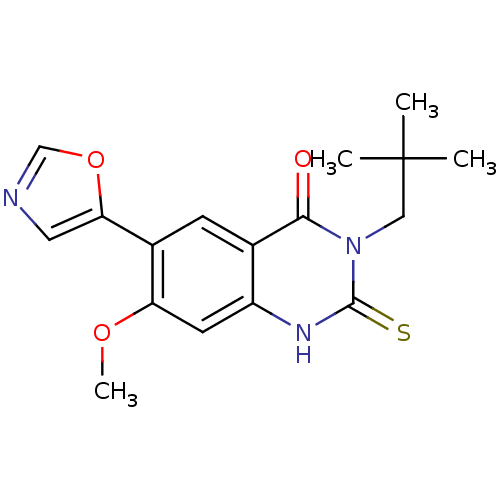
(3-(2,2-Dimethyl-propyl)-7-methoxy-6-oxazol-5-yl-2-...)Show SMILES COc1cc2[nH]c(=S)n(CC(C)(C)C)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C17H19N3O3S/c1-17(2,3)8-20-15(21)10-5-11(14-7-18-9-23-14)13(22-4)6-12(10)19-16(20)24/h5-7,9H,8H2,1-4H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 821 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50174794
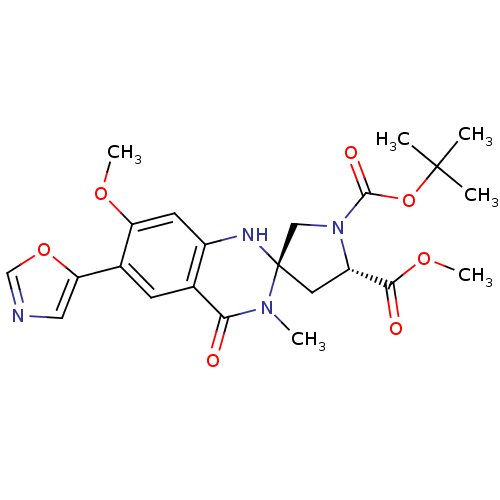
(1-tert-butyl 5-methyl (3R,5S)-7'-methoxy-3'-methyl...)Show SMILES COC(=O)[C@@H]1C[C@@]2(CN1C(=O)OC(C)(C)C)Nc1cc(OC)c(cc1C(=O)N2C)-c1cnco1 Show InChI InChI=1S/C23H28N4O7/c1-22(2,3)34-21(30)27-11-23(9-16(27)20(29)32-6)25-15-8-17(31-5)14(18-10-24-12-33-18)7-13(15)19(28)26(23)4/h7-8,10,12,16,25H,9,11H2,1-6H3/t16-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 948 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibitory activity against IMPDH II |
Bioorg Med Chem Lett 15: 5335-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.108
BindingDB Entry DOI: 10.7270/Q2CZ36P8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50160376
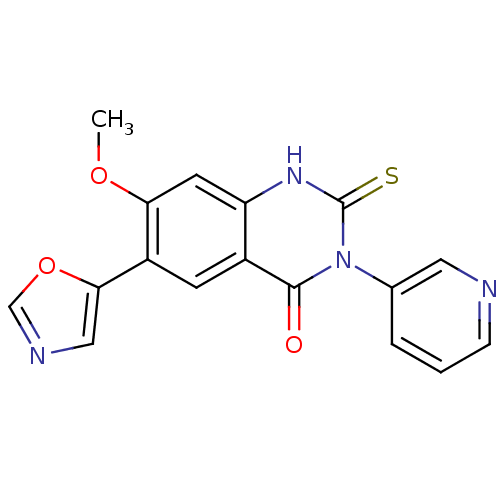
(7-Methoxy-6-oxazol-5-yl-3-pyridin-3-yl-2-thioxo-2,...)Show SMILES COc1cc2[nH]c(=S)n(-c3cccnc3)c(=O)c2cc1-c1cnco1 Show InChI InChI=1S/C17H12N4O3S/c1-23-14-6-13-11(5-12(14)15-8-19-9-24-15)16(22)21(17(25)20-13)10-3-2-4-18-7-10/h2-9H,1H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R and D
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against inosine-5'-monophosphate dehydrogenase |
Bioorg Med Chem Lett 15: 751-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.015
BindingDB Entry DOI: 10.7270/Q2RN37CZ |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525923

(US11185100, TABLE 6.1)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](OP(O)(O)=O)[C@@H](COP(O)(O)=O)O1 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525923

(US11185100, TABLE 6.1)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](OP(O)(O)=O)[C@@H](COP(O)(O)=O)O1 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525931
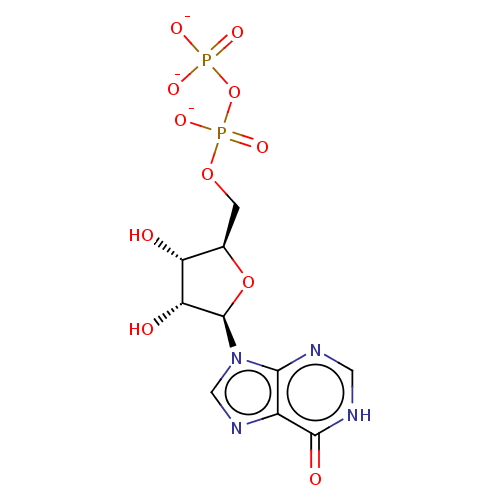
(US11185100, TABLE 6.2)Show SMILES O[C@@H]1[C@@H](COP([O-])(=O)OP([O-])([O-])=O)O[C@H]([C@@H]1O)n1cnc2c1nc[nH]c2=O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525941

(US11185100, TABLE 6.3)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@H](O)C1O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525941

(US11185100, TABLE 6.3)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@H](O)C1O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525941

(US11185100, TABLE 6.3)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@H](O)C1O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525941

(US11185100, TABLE 6.3)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@H](O)C1O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM, and the concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525945

(US11185100, TABLE 6.4)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)OC([C@@H]1O)n1cnc2c1[nH]cnc2=O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525945

(US11185100, TABLE 6.4)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)OC([C@@H]1O)n1cnc2c1[nH]cnc2=O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525945

(US11185100, TABLE 6.4)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)OC([C@@H]1O)n1cnc2c1[nH]cnc2=O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525945

(US11185100, TABLE 6.4)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)OC([C@@H]1O)n1cnc2c1[nH]cnc2=O |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM, and the concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525952

(US11185100, TABLE 6.5)Show SMILES O[C@H]1[C@@H](COP(O)(O)=O)O[C@H](C1O)n1cnc2c(Nc3ccccc3)ncnc12 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525952

(US11185100, TABLE 6.5)Show SMILES O[C@H]1[C@@H](COP(O)(O)=O)O[C@H](C1O)n1cnc2c(Nc3ccccc3)ncnc12 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525952

(US11185100, TABLE 6.5)Show SMILES O[C@H]1[C@@H](COP(O)(O)=O)O[C@H](C1O)n1cnc2c(Nc3ccccc3)ncnc12 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 7.20E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |
Taste receptor type 1 member 1/3
(Homo sapiens (Human)) | BDBM525952

(US11185100, TABLE 6.5)Show SMILES O[C@H]1[C@@H](COP(O)(O)=O)O[C@H](C1O)n1cnc2c(Nc3ccccc3)ncnc12 |r| | UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration of alanine is 20 mM, and the concentration of IMP is 0.2 mM. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KP859X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data