

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50092393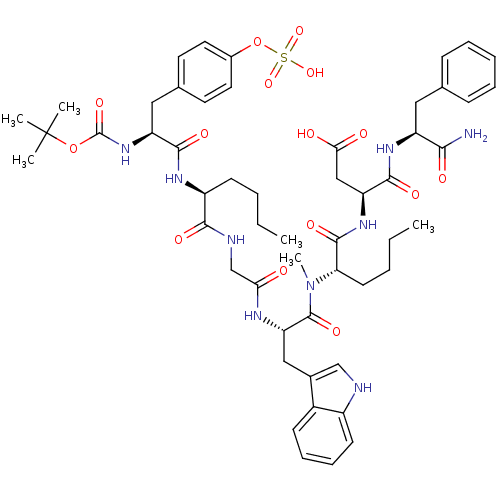 (3-(2-{[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50092405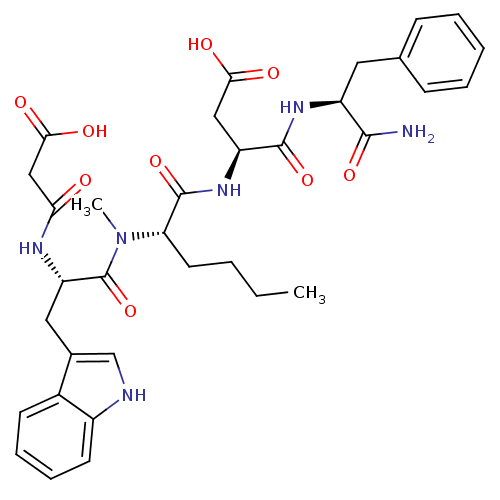 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50016504 ((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranes | Bioorg Med Chem Lett 14: 369-72 (2003) BindingDB Entry DOI: 10.7270/Q2RJ4K1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50092393 (3-(2-{[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-su...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50449787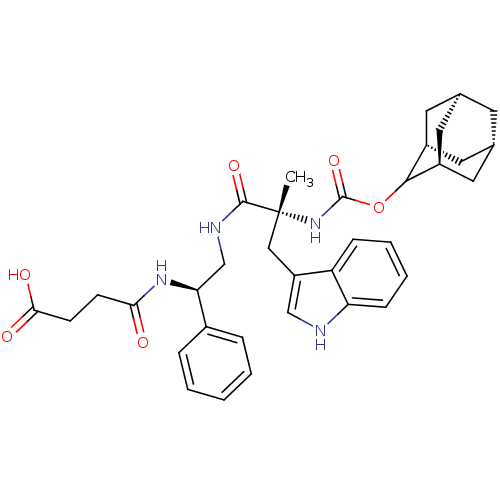 (CHEMBL2062154 | PD-134308) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for the affinity against Cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 40: 3947-56 (1998) Article DOI: 10.1021/jm970439a BindingDB Entry DOI: 10.7270/Q27H1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21444 (N-Benylpiperazine derivative, 23m | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 30 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21439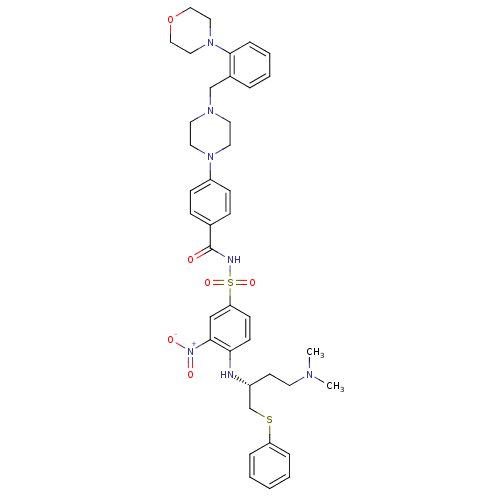 (N-Benylpiperazine derivative, 23g | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 430 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21421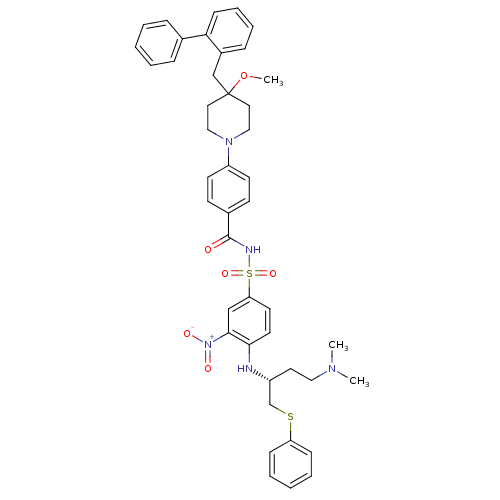 (4-Alkyl-4-methoxypiperidine derivative, 8m | N-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | <60 | n/a | 35 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21449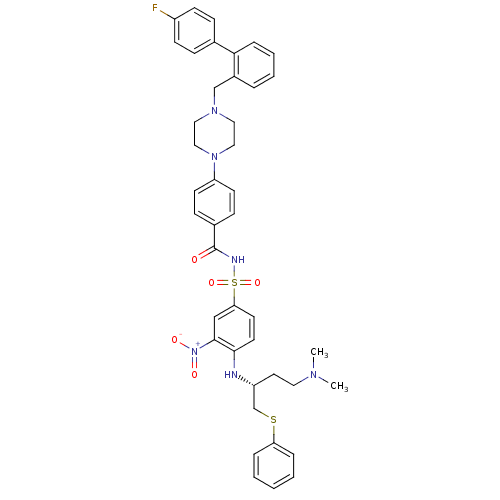 (N-Benylpiperazine derivative, 23q | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 130 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21441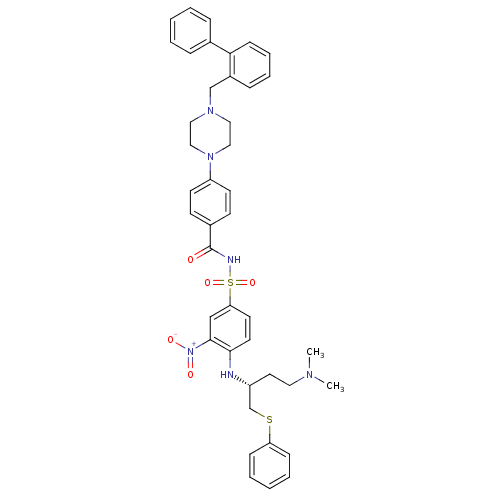 (N-Benylpiperazine derivative, 23i | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 18 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21450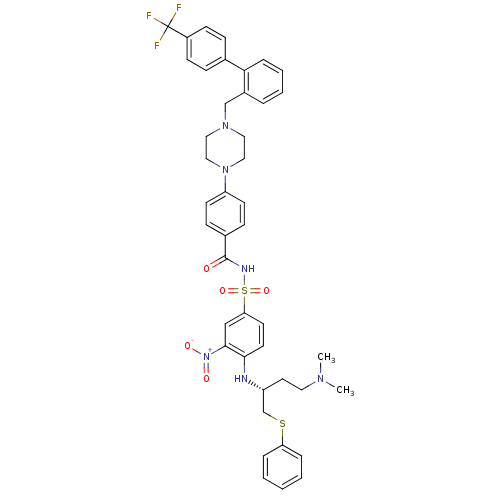 (N-Benylpiperazine derivative, 23r | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 40 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 30 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21420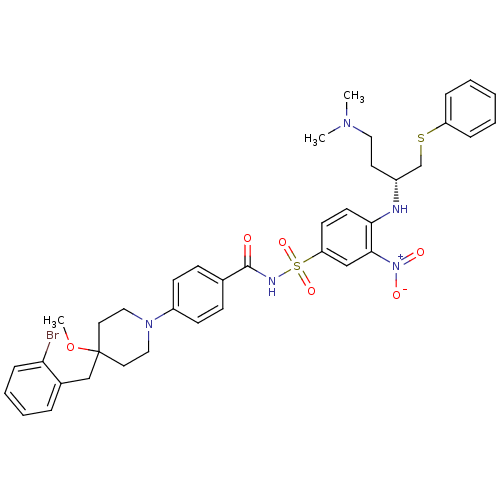 (4-Alkyl-4-methoxypiperidine derivative, 8l | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | <60 | n/a | 100 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21436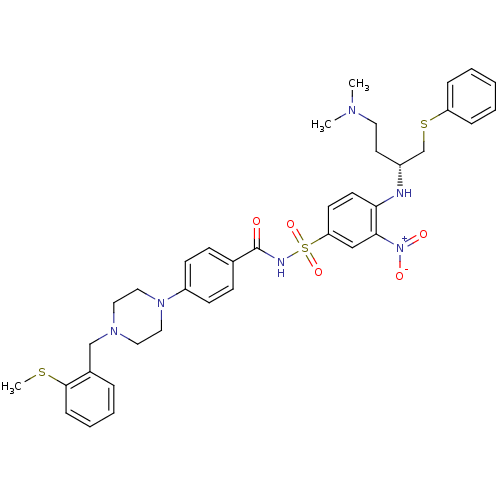 (N-Benylpiperazine derivative, 23d | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 95 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21445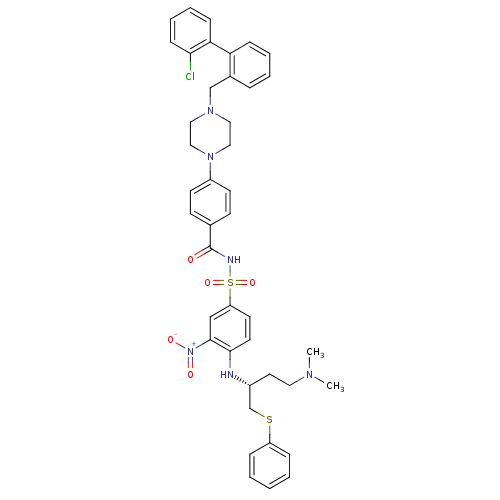 (4-(4-{[2-(2-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 56 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21440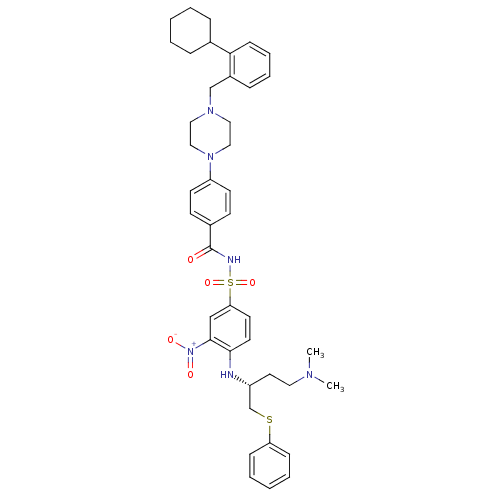 (4-{4-[(2-cyclohexylphenyl)methyl]piperazin-1-yl}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 61 | n/a | 340 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21414 (4-Alkyl-4-methoxypiperidine derivative, 8f | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 62 | n/a | 230 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21418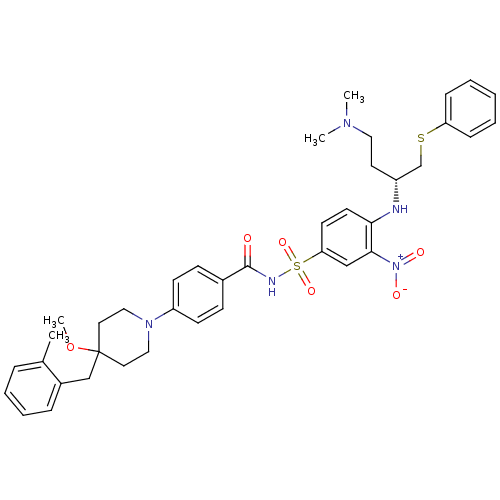 (4-Alkyl-4-methoxypiperidine derivative, 8j | N-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 71 | n/a | 150 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21435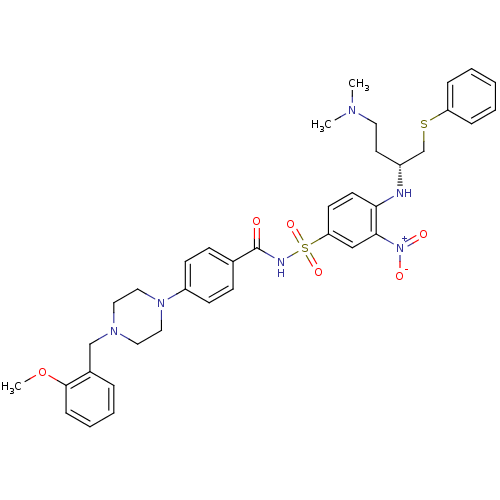 (N-Benylpiperazine derivative, 23c | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 80 | n/a | 390 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21442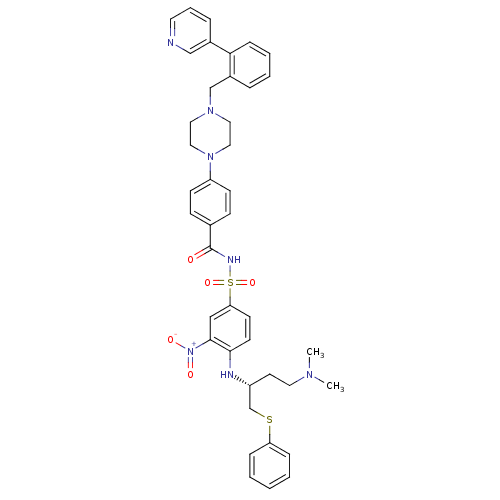 (N-Benylpiperazine derivative, 23k | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 81 | n/a | 800 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21432 (4-Piperidinebenzylidene derivative, 10k | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 83 | n/a | 160 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21434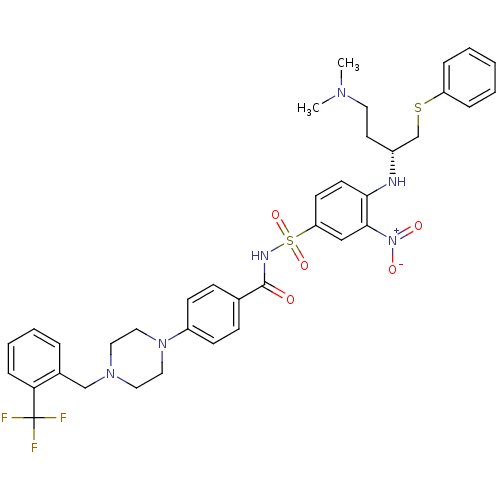 (N-Benylpiperazine derivative, 23j | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 85 | n/a | 390 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21446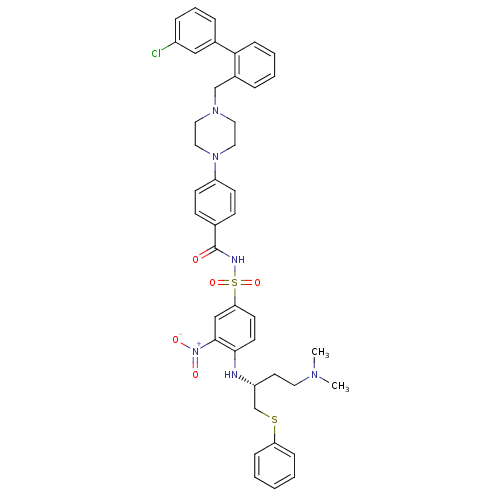 (4-(4-{[2-(3-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 140 | n/a | 180 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21415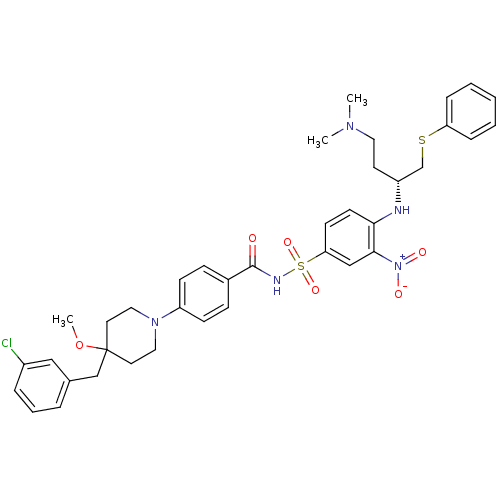 (4-Alkyl-4-methoxypiperidine derivative, 8g | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 170 | n/a | 250 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21443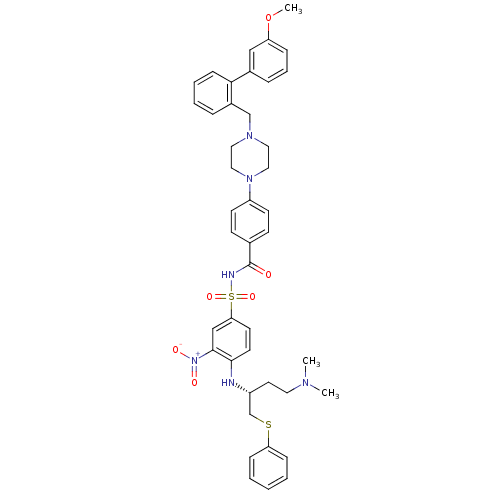 (N-Benylpiperazine derivative, 23l | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 178 | n/a | 380 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21426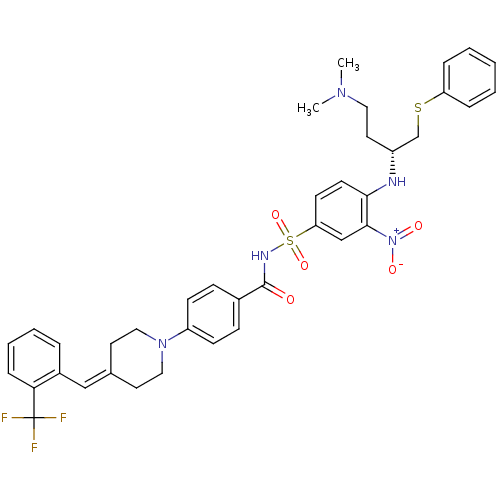 (4-Piperidinebenzylidene derivative, 10e | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 300 | n/a | 150 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21425 (4-Piperidinebenzylidene derivative, 10d | 4-{4-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 510 | n/a | 620 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21429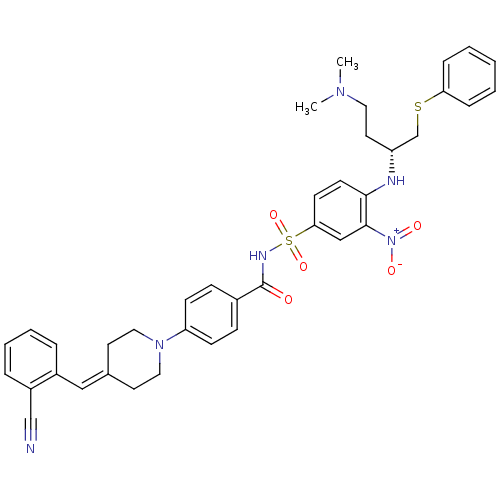 (4-Piperidinebenzylidene derivative, 10h | 4-{4-[(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 520 | n/a | 1.10E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21424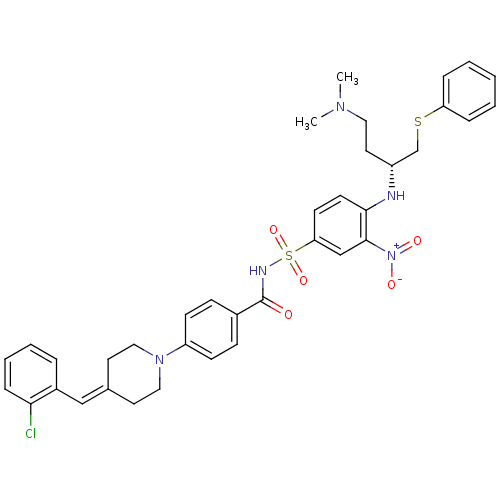 (4-Piperidinebenzylidene derivative, 10c | 4-{4-[(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 700 | n/a | 350 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21423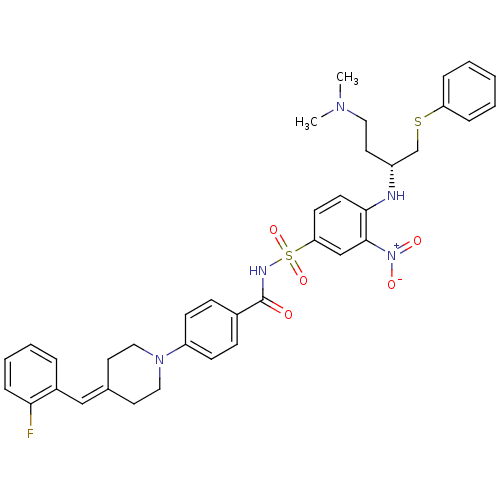 (4-Piperidinebenzylidene derivative, 10b | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 750 | n/a | 250 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50061266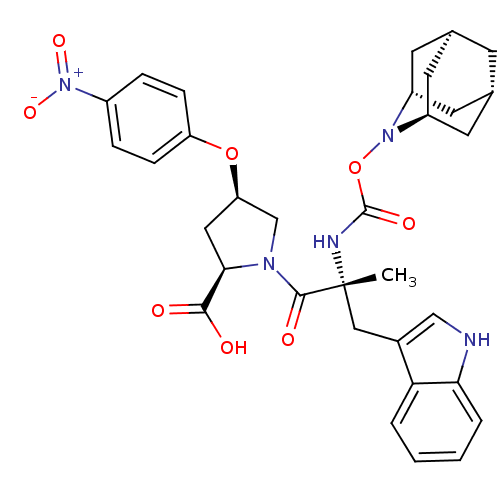 ((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity (affinity state 1) for Cholecystokinin type B receptor, was determined using CHO cells | J Med Chem 40: 3947-56 (1998) Article DOI: 10.1021/jm970439a BindingDB Entry DOI: 10.7270/Q27H1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50181875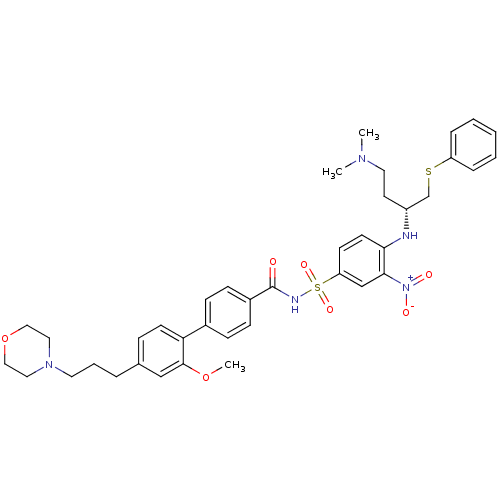 (4-((R)-3-dimethylamino-1-phenylsulfanylmethylpropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL by fluorescence polarization assay | J Med Chem 49: 1165-81 (2006) Article DOI: 10.1021/jm050754u BindingDB Entry DOI: 10.7270/Q2VM4BT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50181869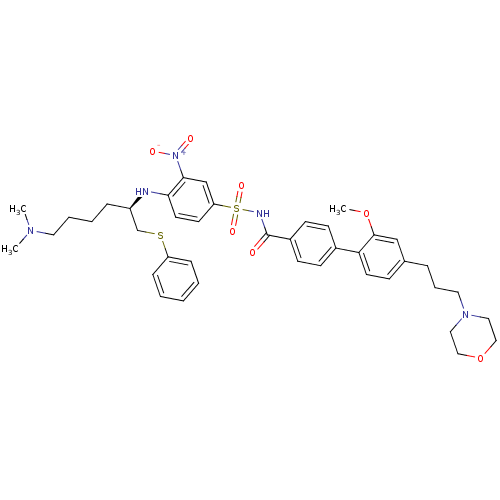 (4-((R)-5-dimethylamino-1-phenylsulfanylmethylpenty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL by fluorescence polarization assay | J Med Chem 49: 1165-81 (2006) Article DOI: 10.1021/jm050754u BindingDB Entry DOI: 10.7270/Q2VM4BT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50181881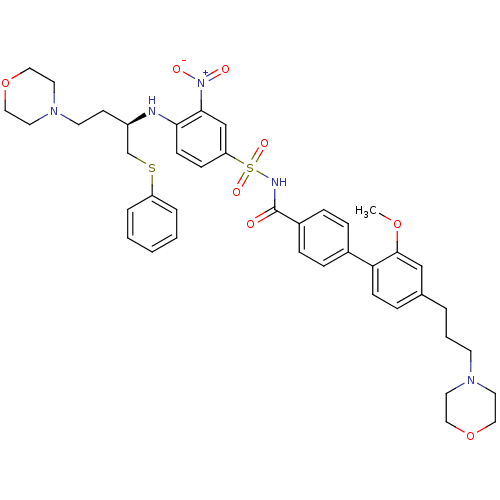 (CHEMBL370837 | N-[2'-methoxy-4'-(3-morpholin-4-ylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL by fluorescence polarization assay | J Med Chem 49: 1165-81 (2006) Article DOI: 10.1021/jm050754u BindingDB Entry DOI: 10.7270/Q2VM4BT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21405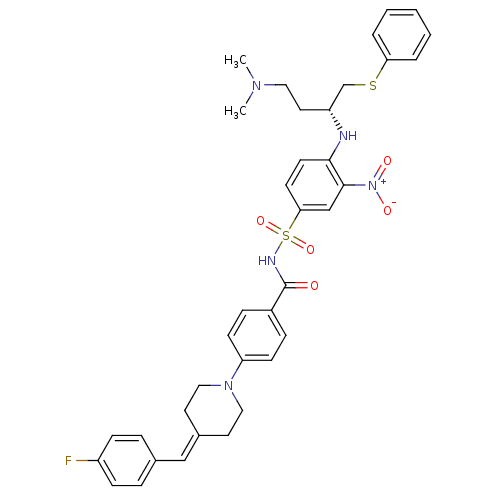 (4-Piperidinebenzylidene derivative, 10a | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | n/a | n/a | 380 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50092408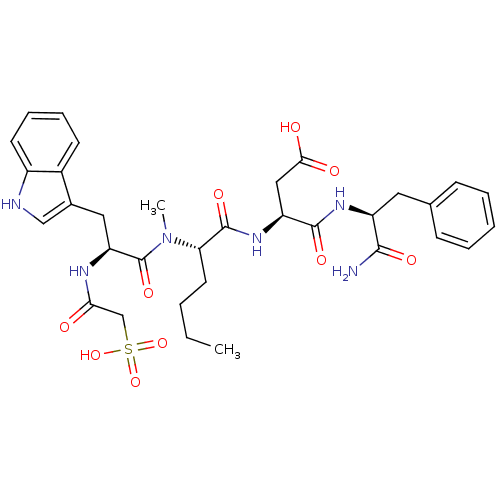 (CHEMBL120150 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21438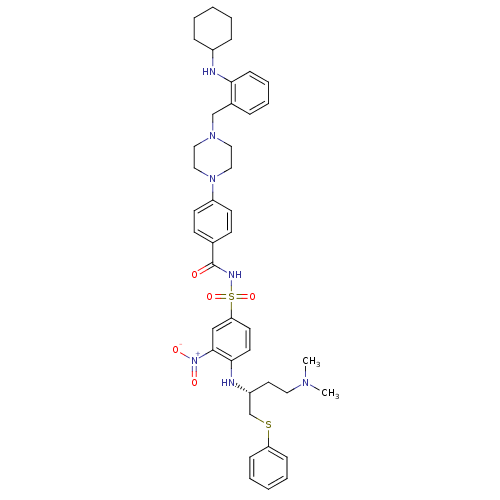 (4-(4-{[2-(cyclohexylamino)phenyl]methyl}piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | 370 | n/a | 660 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21428 (4-Piperidinebenzylidene derivative, 10g | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | -52.6 | 760 | n/a | 300 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranes | Bioorg Med Chem Lett 14: 369-72 (2003) BindingDB Entry DOI: 10.7270/Q2RJ4K1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50092405 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Ability to displace 1 nM [3H]pCCK-8 from rat Cholecystokinin type B receptor stably expressing in CHO cells | Bioorg Med Chem Lett 14: 369-72 (2003) BindingDB Entry DOI: 10.7270/Q2RJ4K1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for binding affinity against CCK1 (cholecystokinin) receptor on guinea pig pancreatic membranes | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50092398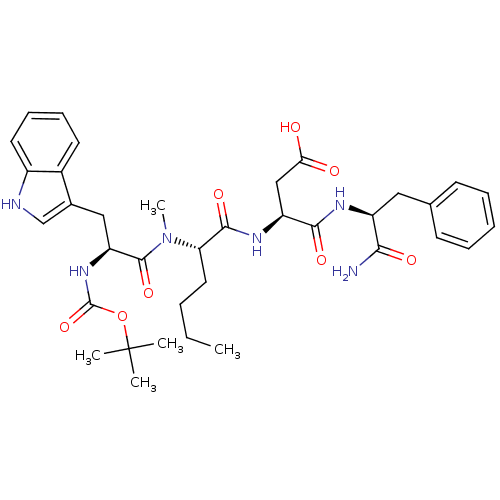 ((S)-3-((S)-2-{[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21424 (4-Piperidinebenzylidene derivative, 10c | 4-{4-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50092405 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranes | Bioorg Med Chem Lett 14: 369-72 (2003) BindingDB Entry DOI: 10.7270/Q2RJ4K1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50092405 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21413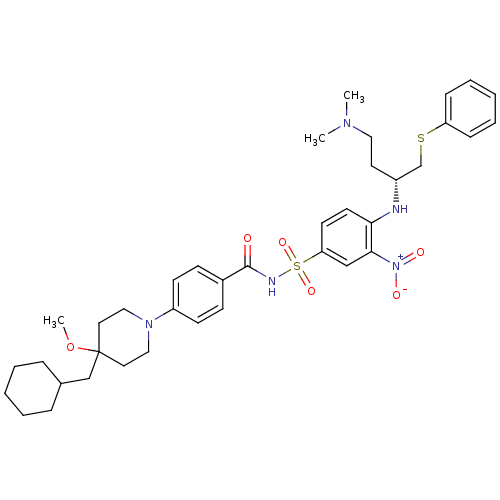 (4-Alkyl-4-methoxypiperidine derivative, 8d | 4-[4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | -51.9 | 300 | n/a | 320 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50181889 (4-((R)-5-amino-1-phenylsulfanylmethylpentylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL by fluorescence polarization assay | J Med Chem 49: 1165-81 (2006) Article DOI: 10.1021/jm050754u BindingDB Entry DOI: 10.7270/Q2VM4BT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3437 total ) | Next | Last >> |