

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50589230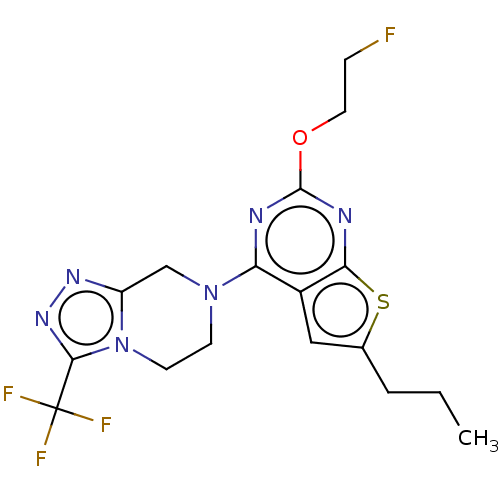 (CHEMBL5181634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50298132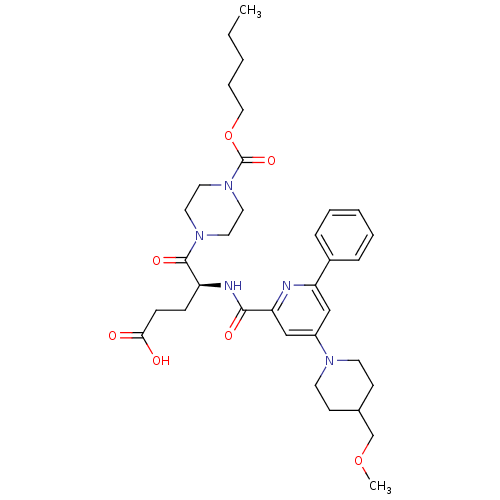 ((4S)4-[({4-[4-(Methoxymethyl)piperidin-1-yl]-6-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397143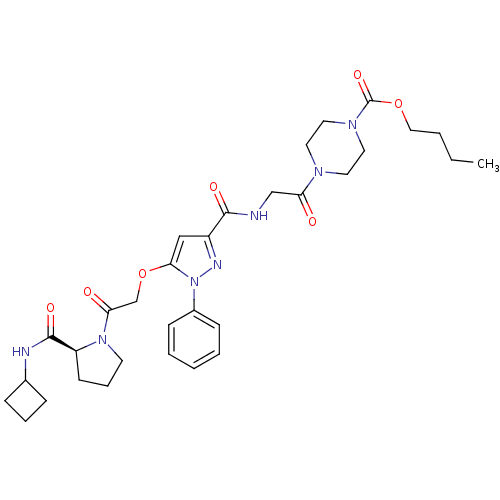 (CHEMBL2172140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50589229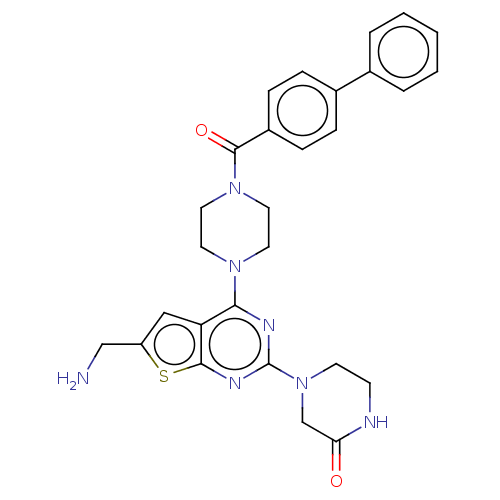 (CHEMBL5203993) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397204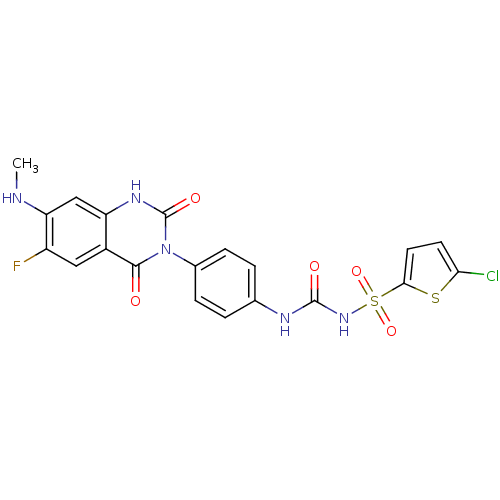 (ELINOGREL) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50268877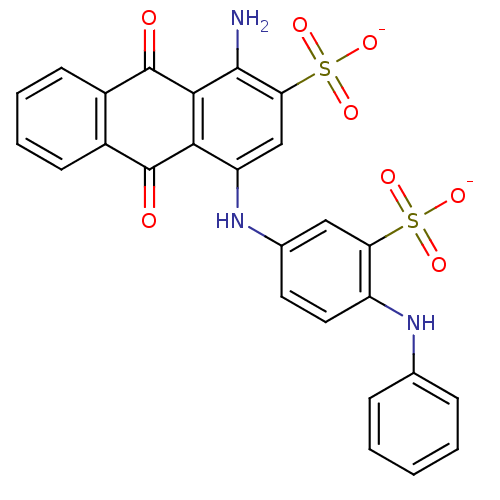 (CHEMBL455536 | Disodium 1-Amino-4-[4-phenylamino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50118242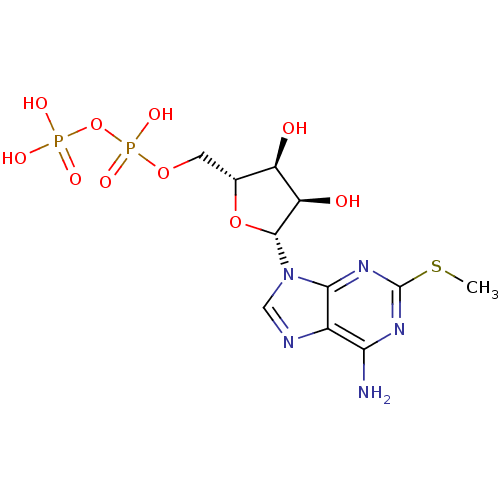 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50118225 (ARL 69931MX | Adenosine triphosphate derivative | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Capital Normal University Curated by ChEMBL | Assay Description Inhibition of human dihydrofolate reductase by spectrophotometric analysis | Eur J Med Chem 64: 401-9 (2013) Article DOI: 10.1016/j.ejmech.2013.04.017 BindingDB Entry DOI: 10.7270/Q20P11F4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397205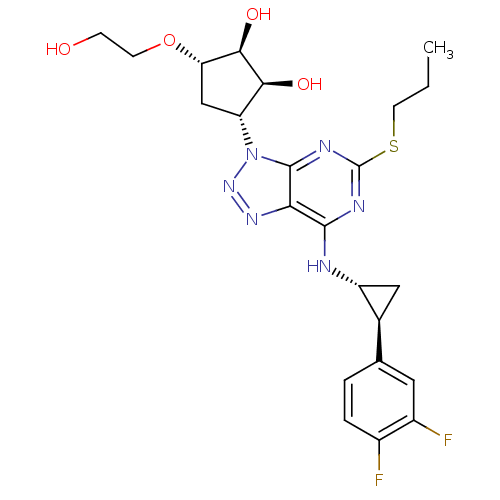 (AR-C126532XX | AZD-6140 | AZD6140 | BRILINTA | TIC...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003386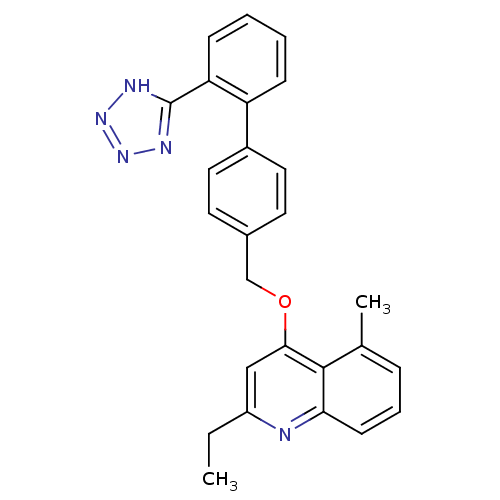 (2-Ethyl-5-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003392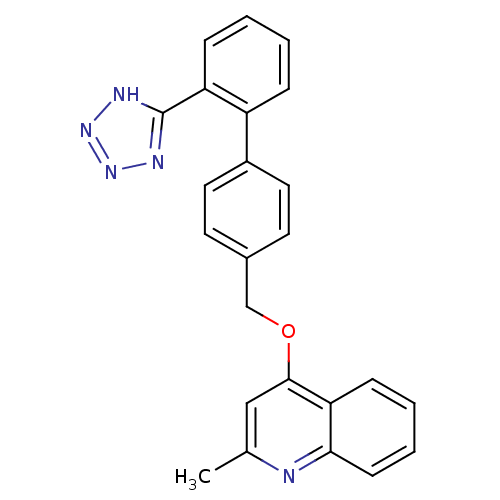 (2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100247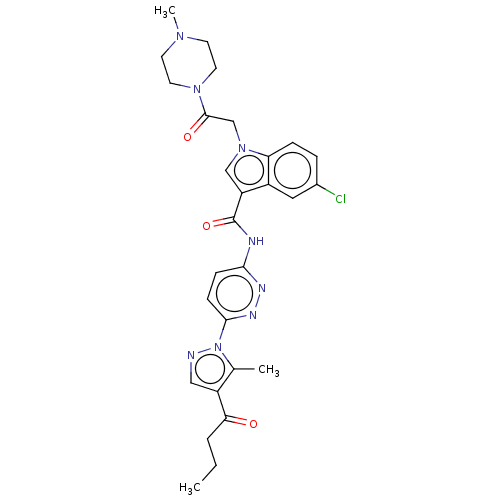 (CHEMBL3325891) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50406795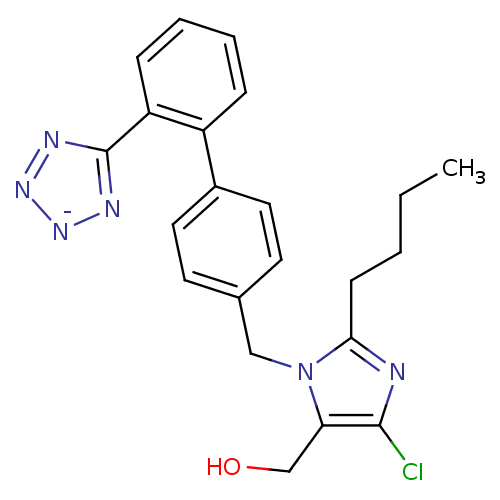 (Cozaar | LOSARTAN POTASSIUM) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003400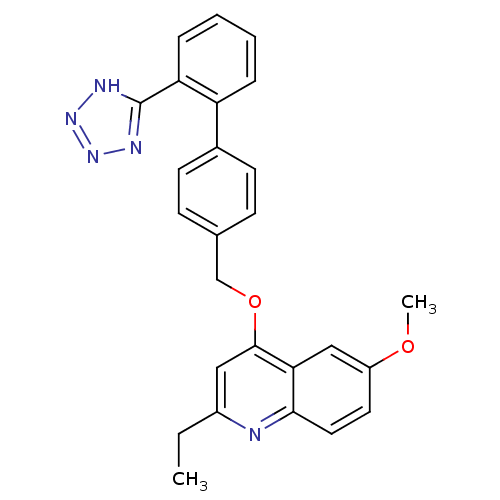 (2-Ethyl-6-methoxy-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003377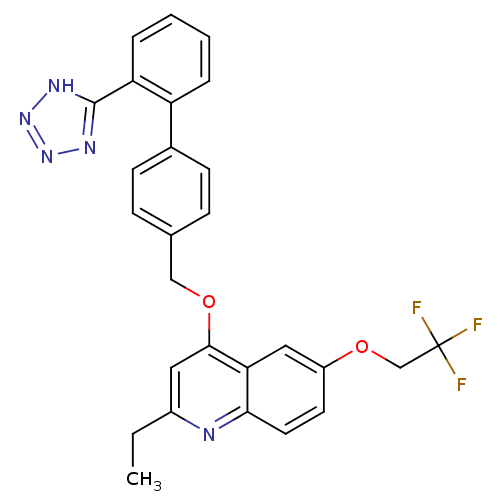 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003403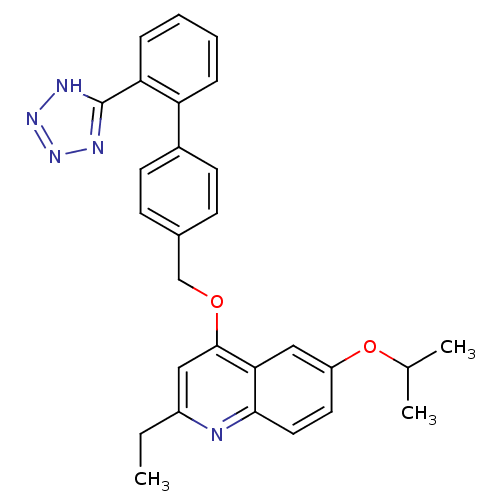 (2-Ethyl-6-isopropoxy-4-[2'-(1H-tetrazol-5-yl)-biph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003387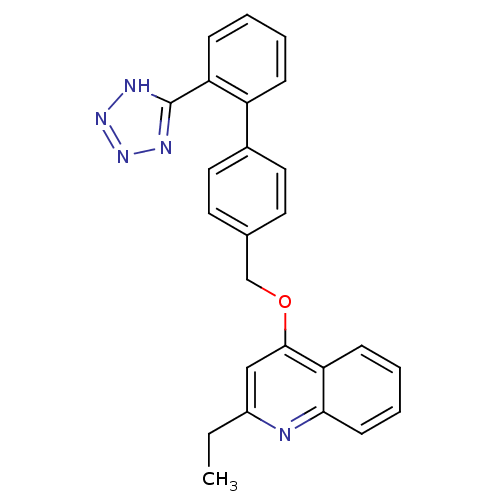 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003394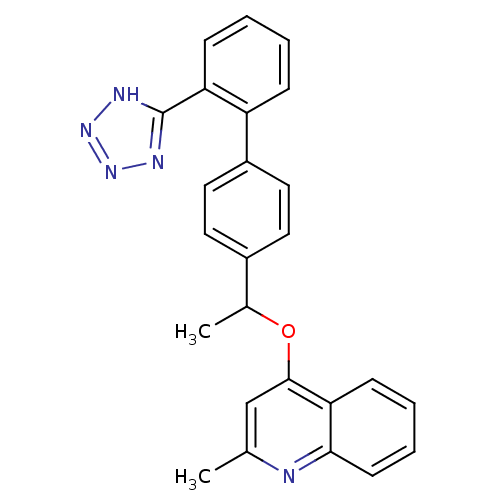 (2-Methyl-4-{1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003376 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003398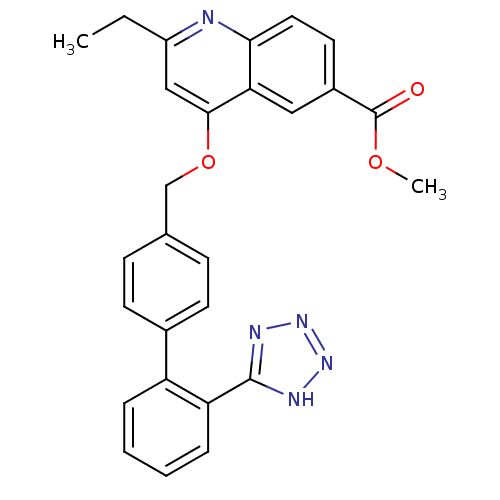 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003382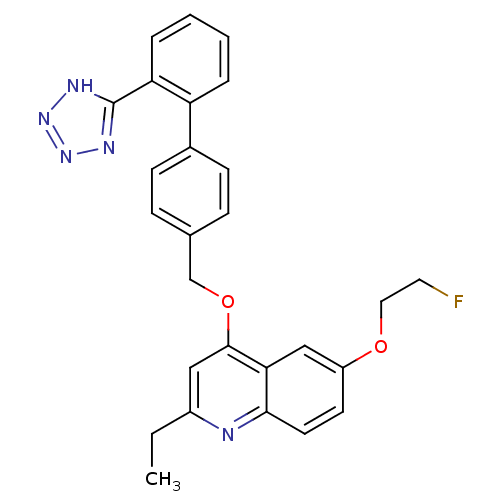 (2-Ethyl-6-(2-fluoro-ethoxy)-4-[2'-(1H-tetrazol-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50005340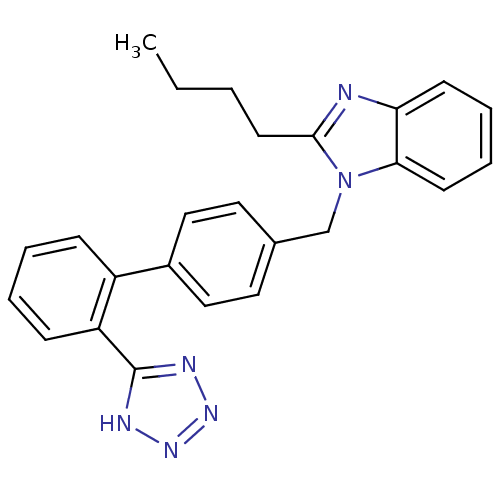 (2-Butyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003404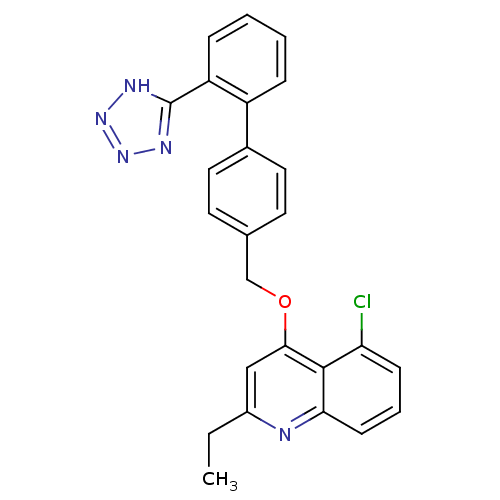 (5-Chloro-2-ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003380 (2-Ethyl-7-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003384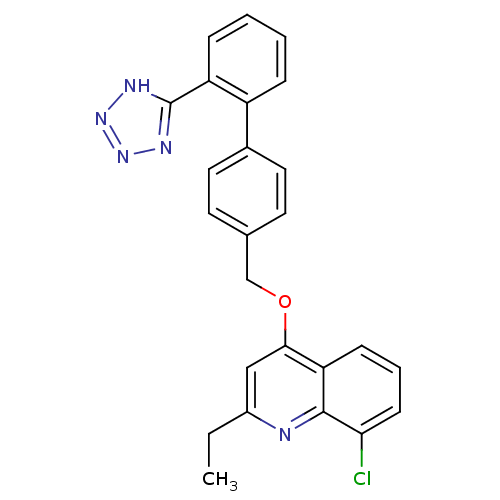 (8-Chloro-2-ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003395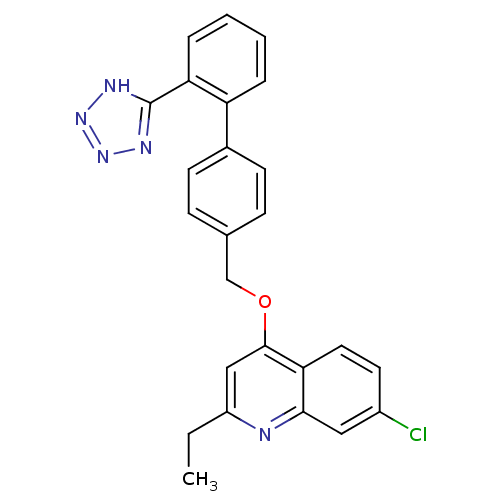 (7-Chloro-2-ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003381 (4'-(2-Ethyl-quinolin-4-yloxymethyl)-biphenyl-2-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003378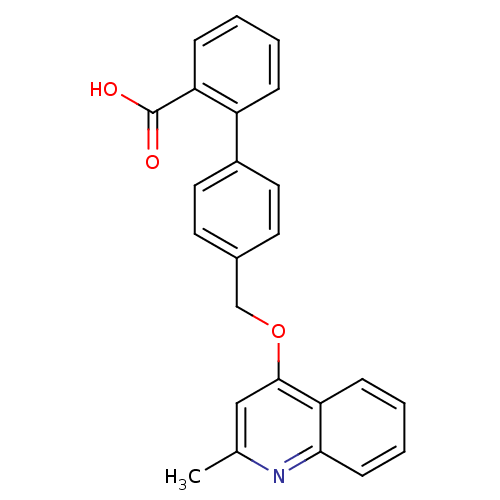 (4'-(2-Methyl-quinolin-4-yloxymethyl)-biphenyl-2-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003379 (2-Ethyl-7-methoxy-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18795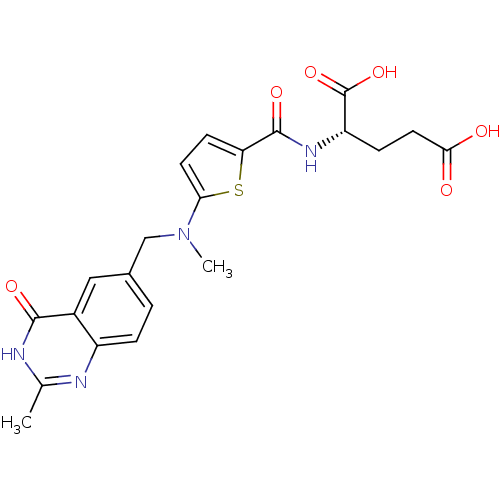 ((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Capital Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged thymidylate synthase assessed as N5,N10-methylenetetrahydrofolate oxidation to dihydrofolate after 20 mins... | Eur J Med Chem 64: 401-9 (2013) Article DOI: 10.1016/j.ejmech.2013.04.017 BindingDB Entry DOI: 10.7270/Q20P11F4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003391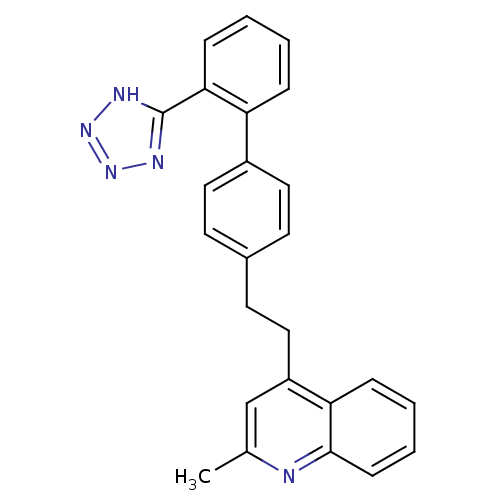 (2-Methyl-4-{2-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003389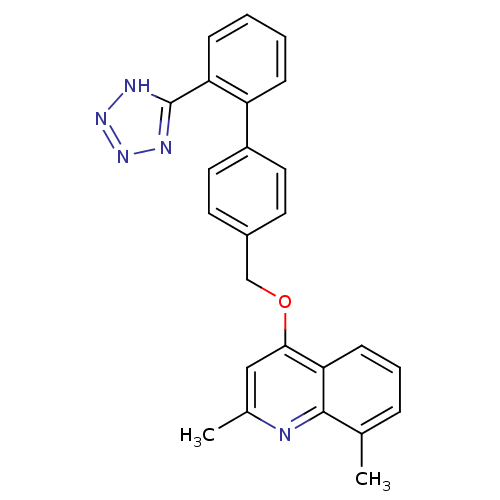 (2,8-Dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003402 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003406 (2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003399 (4'-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003390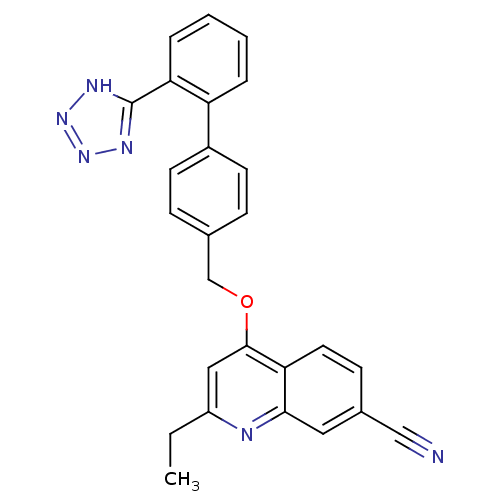 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003374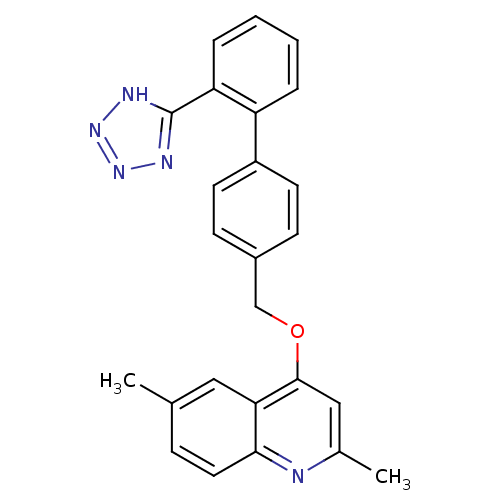 (2,6-Dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50003399 (4'-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003393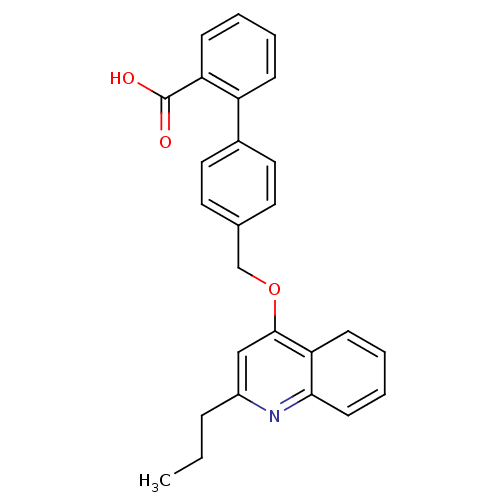 (4'-(2-Propyl-quinolin-4-yloxymethyl)-biphenyl-2-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230428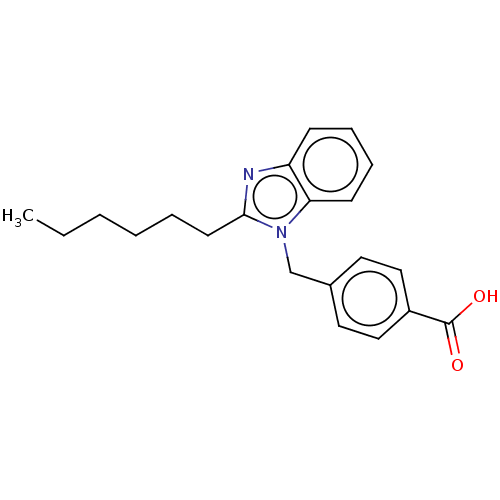 (CHEMBL352376) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230436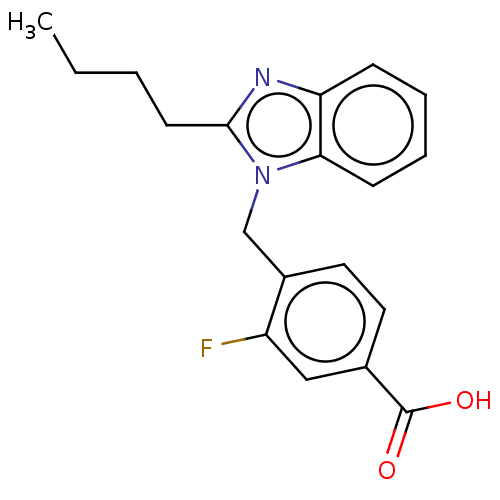 (CHEMBL424372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003388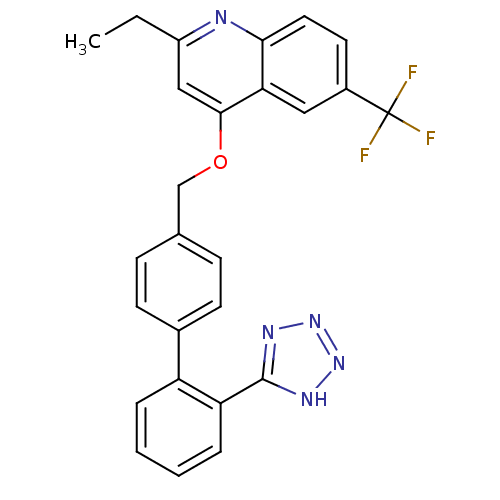 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003396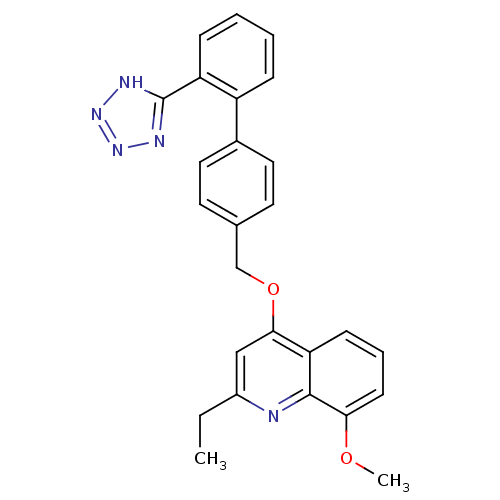 (2-Ethyl-8-methoxy-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003375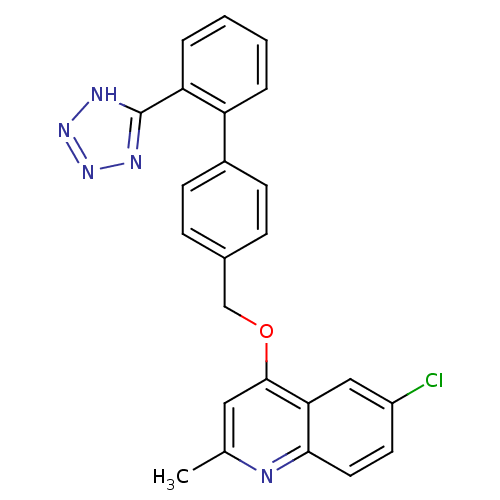 (6-Chloro-2-methyl-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003397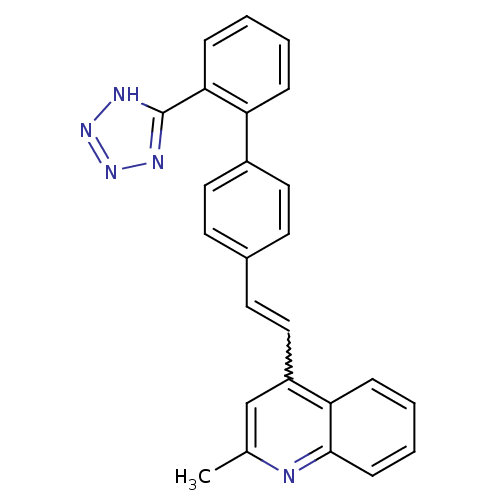 (2-Methyl-4-{2-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230438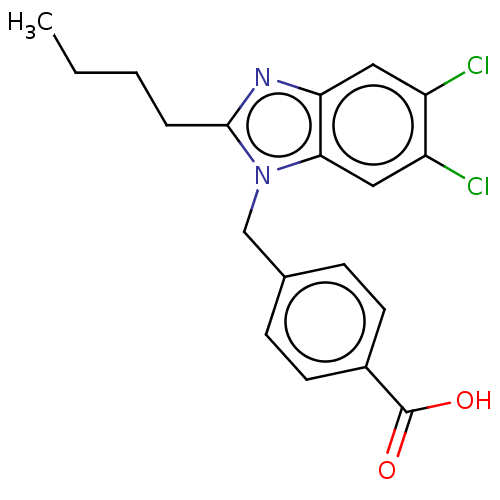 (CHEMBL171530) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50434818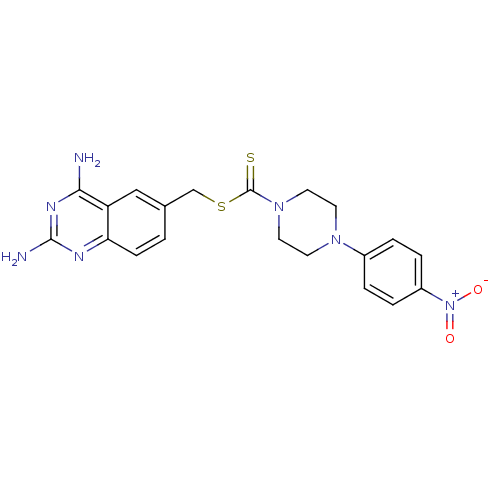 (CHEMBL2387094) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Capital Normal University Curated by ChEMBL | Assay Description Inhibition of human dihydrofolate reductase by spectrophotometric analysis | Eur J Med Chem 64: 401-9 (2013) Article DOI: 10.1016/j.ejmech.2013.04.017 BindingDB Entry DOI: 10.7270/Q20P11F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50589231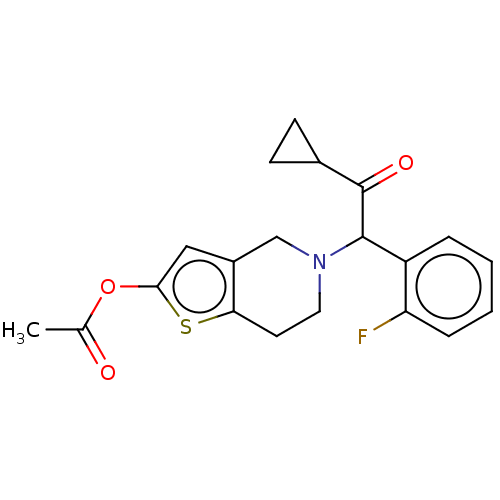 (CHEBI:87723 | Efient | LY-640315 | NSC-759625 | PR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128837 BindingDB Entry DOI: 10.7270/Q2765K8C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230439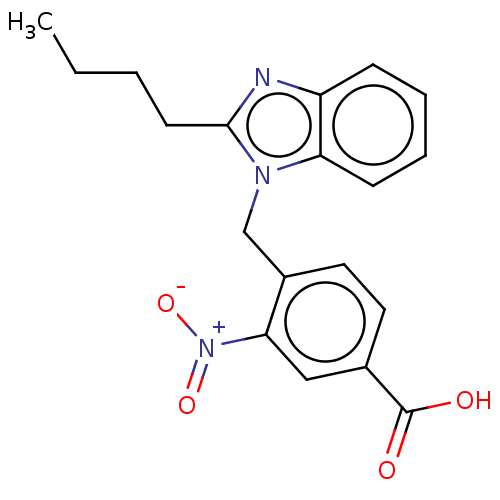 (CHEMBL172344) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |