

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50529214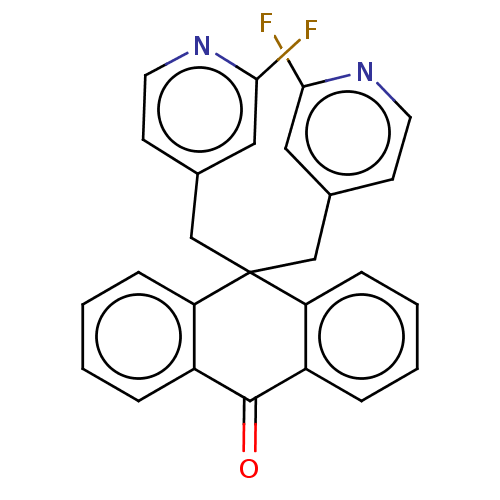 (CHEMBL343822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1A adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529212 (CHEMBL4471453) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein polybromo-1 (Homo sapiens (Human)) | BDBM50529212 (CHEMBL4471453) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of PBR receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529214 (CHEMBL343822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50529213 (CHEMBL4465132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of D3 receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529213 (CHEMBL4465132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502780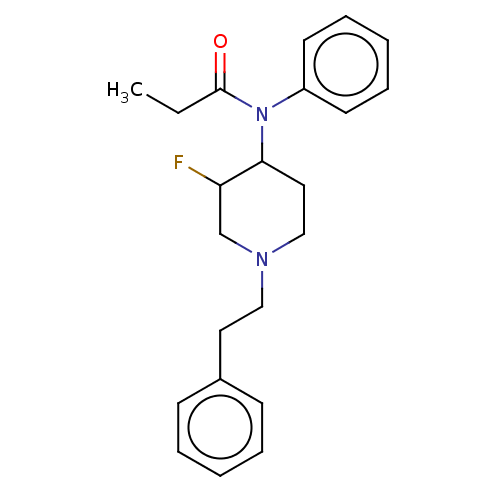 (CHEMBL4471584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502783 (CHEMBL4468844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502780 (CHEMBL4471584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502783 (CHEMBL4468844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50503927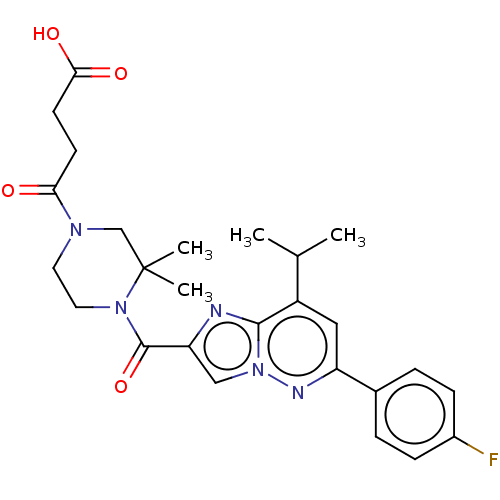 (CHEMBL4470628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human EAhy926 cells assessed as inhibition of trypsin-induced intracellular calcium mobilization preincubated for 15 m... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069751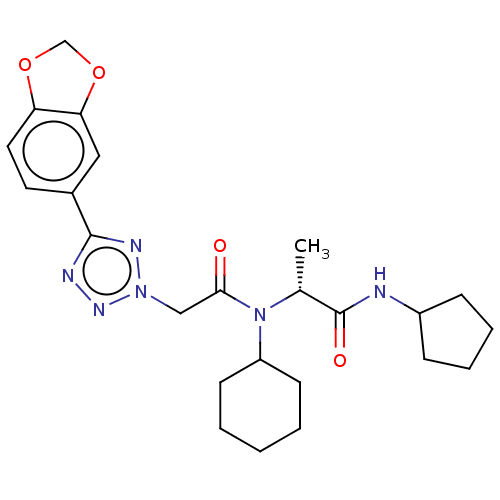 (CHEMBL2356114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in uptake of [3H]CE from [3H]CE-HDL by by liquid scintillation count... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human MDA-MB-231 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069751 (CHEMBL2356114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-B1 overexpressed in CHO cells assessed as inhibition of [3H]cholesteryl ester uptake into cells after 2 to 3 hrs by liquid sci... | Bioorg Med Chem Lett 25: 2100-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.073 BindingDB Entry DOI: 10.7270/Q2P84DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50276409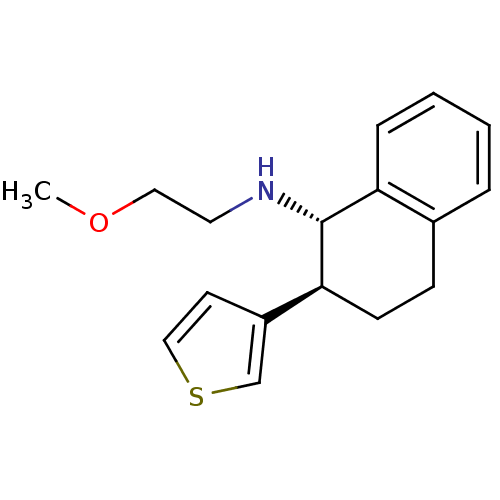 ((1S,2S)-N-(2-methoxyethyl)-2-(thiophen-3-yl)-1,2,3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Displacement of [125I]FK33824 from human cloned mu opioid receptor | Bioorg Med Chem Lett 19: 1228-32 (2009) Article DOI: 10.1016/j.bmcl.2008.12.095 BindingDB Entry DOI: 10.7270/Q2GT5N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502786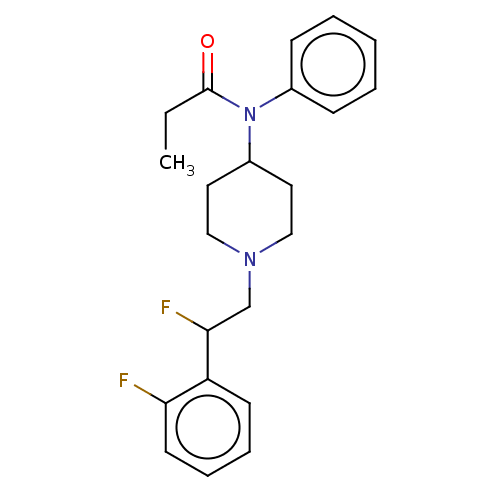 (CHEMBL4441320) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502783 (CHEMBL4468844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502784 (CHEMBL4458454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502780 (CHEMBL4471584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503928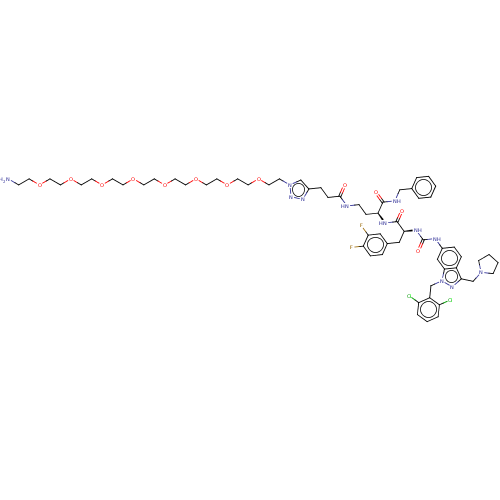 (CHEMBL4459024) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human MDA-MB-231 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502785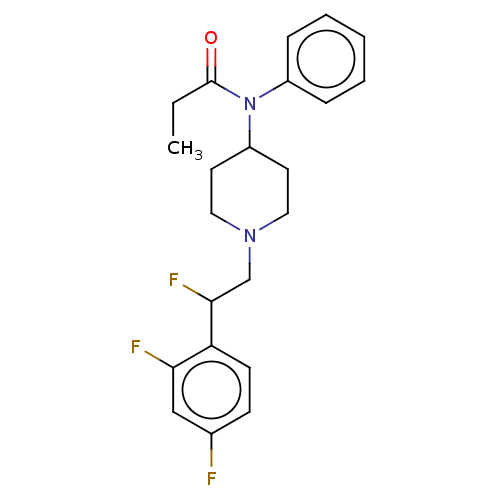 (CHEMBL4550454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069751 (CHEMBL2356114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in transfer of fluorescent lipid DiI from human HDL particles into c... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50088281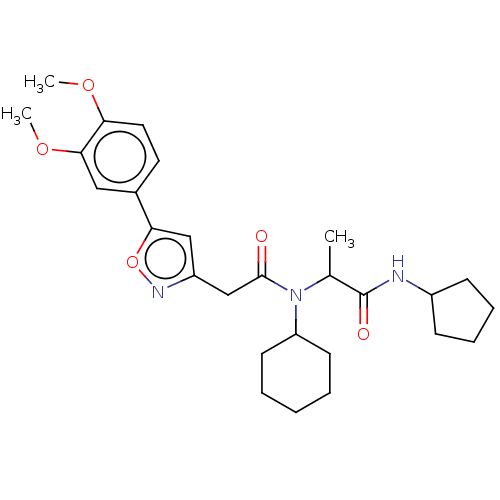 (CHEMBL3427475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in transfer of fluorescent lipid DiI from human HDL particles into c... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502782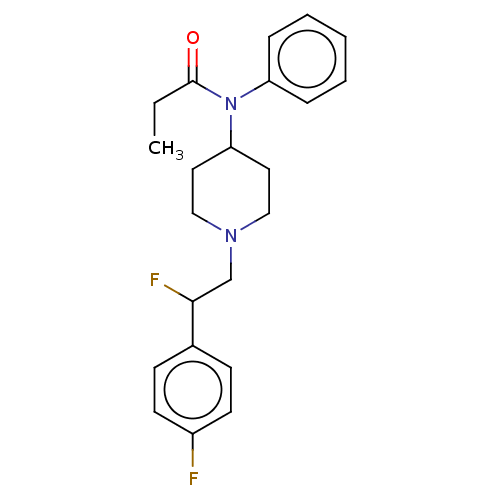 (CHEMBL4516683) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 6.5 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50088195 (CHEMBL3427497) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in transfer of fluorescent lipid DiI from human HDL particles into c... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50088267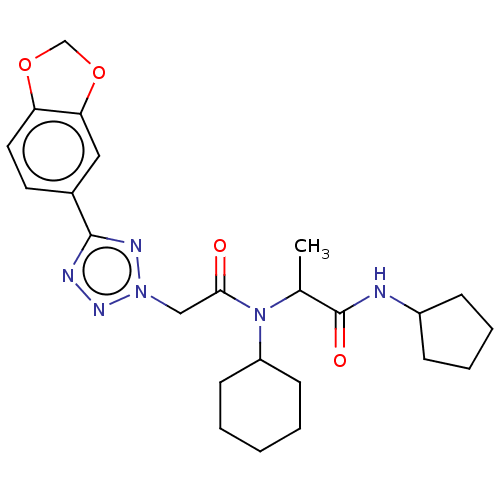 (CHEMBL3427489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in transfer of fluorescent lipid DiI from human HDL particles into c... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503926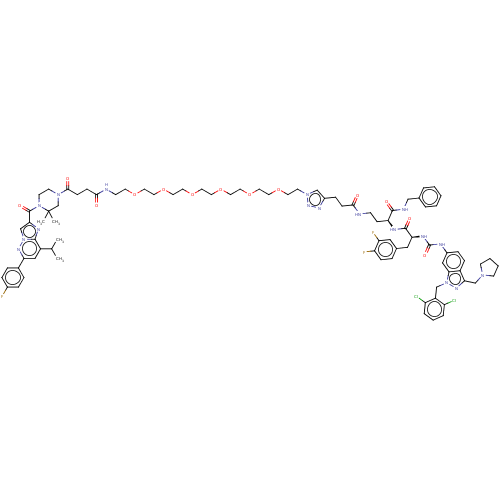 (CHEMBL4472608) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50461680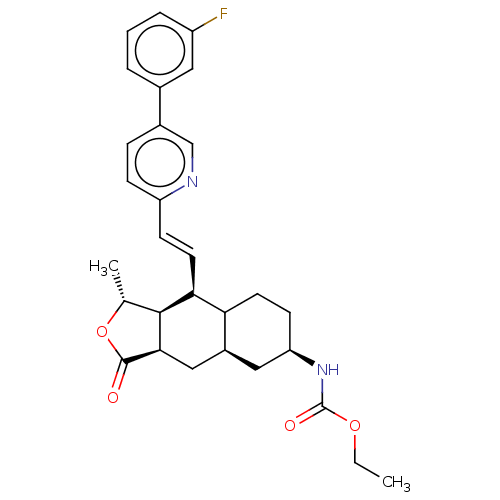 (CHEMBL4226439) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50461681 (Atopaxar | E-5555 | E5555 | ER-172594-00) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50461680 (CHEMBL4226439) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50461681 (Atopaxar | E-5555 | E5555 | ER-172594-00) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization pretreated for 15 ... | Bioorg Med Chem 26: 2514-2529 (2018) Article DOI: 10.1016/j.bmc.2018.04.016 BindingDB Entry DOI: 10.7270/Q23R0WHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503924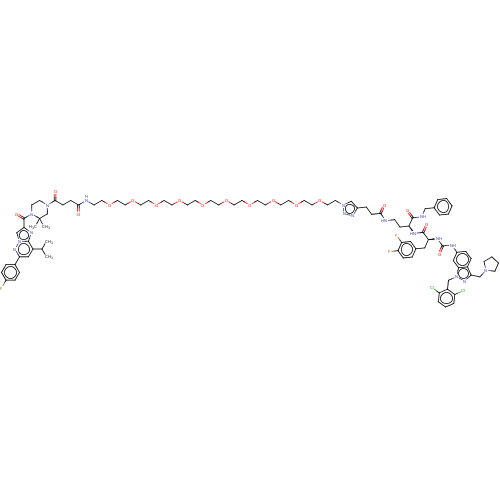 (CHEMBL4437508) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529214 (CHEMBL343822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069719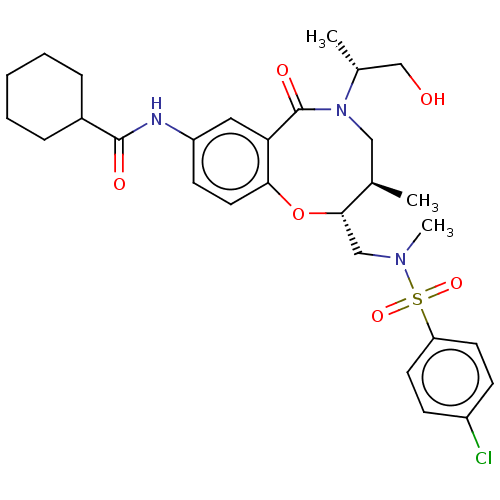 (CHEMBL2358771) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-B1 overexpressed in CHO cells assessed as inhibition of transfer of the fluorescent lipid DiI from HDL particles to cells afte... | Bioorg Med Chem Lett 25: 2100-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.073 BindingDB Entry DOI: 10.7270/Q2P84DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50088283 (CHEMBL1478888) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in transfer of fluorescent lipid DiI from human HDL particles into c... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529215 (CHEBI:35046 | CHEMBL342375) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50276496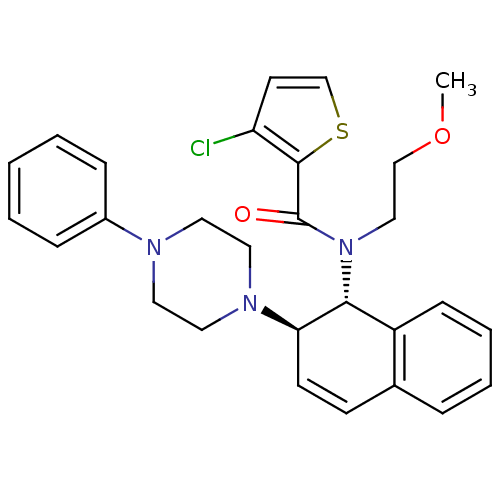 (3-chloro-N-(2-methoxyethyl)-N-((1R,2R)-2-(4-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Displacement of [125I]FK33824 from human cloned mu opioid receptor | Bioorg Med Chem Lett 19: 1228-32 (2009) Article DOI: 10.1016/j.bmcl.2008.12.095 BindingDB Entry DOI: 10.7270/Q2GT5N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069697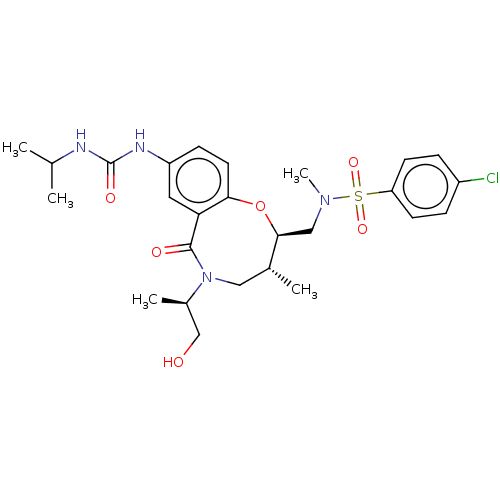 (CHEMBL2356172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-B1 overexpressed in CHO cells assessed as inhibition of transfer of the fluorescent lipid DiI from HDL particles to cells afte... | Bioorg Med Chem Lett 25: 2100-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.073 BindingDB Entry DOI: 10.7270/Q2P84DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50276363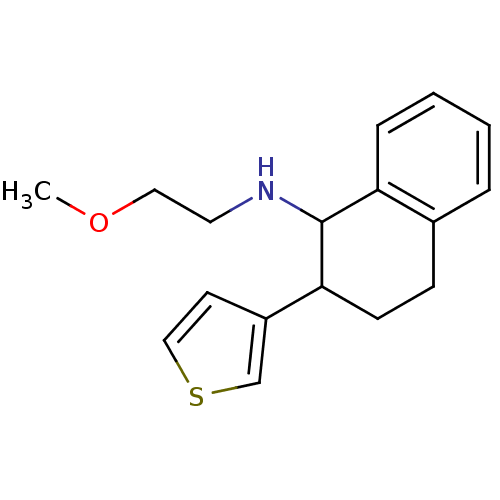 ((+/-)-N-(2-methoxyethyl)-2-(thiophen-3-yl)-1,2,3,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Displacement of [125I]FK33824 from human cloned mu opioid receptor | Bioorg Med Chem Lett 19: 1228-32 (2009) Article DOI: 10.1016/j.bmcl.2008.12.095 BindingDB Entry DOI: 10.7270/Q2GT5N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502784 (CHEMBL4458454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50502785 (CHEMBL4550454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at Gi-coupled mu opioid receptor (unknown origin) expressed in HEK293T cells assessed as inhibition of cAMP production at pH 7.4 mea... | ACS Med Chem Lett 10: 1353-1356 (2019) Article DOI: 10.1021/acsmedchemlett.9b00335 BindingDB Entry DOI: 10.7270/Q2PZ5D3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50069714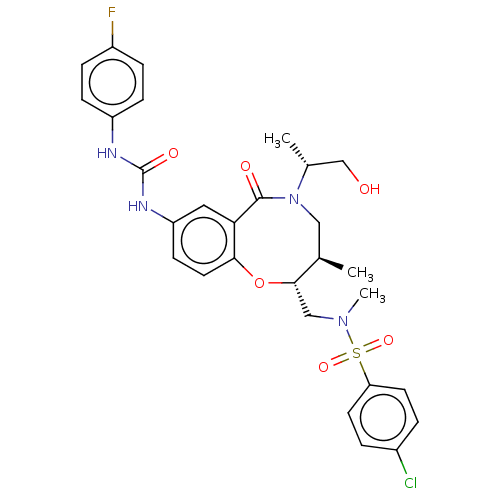 (CHEMBL2356554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-B1 overexpressed in CHO cells assessed as inhibition of transfer of the fluorescent lipid DiI from HDL particles to cells afte... | Bioorg Med Chem Lett 25: 2100-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.073 BindingDB Entry DOI: 10.7270/Q2P84DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503928 (CHEMBL4459024) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 345 total ) | Next | Last >> |