

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343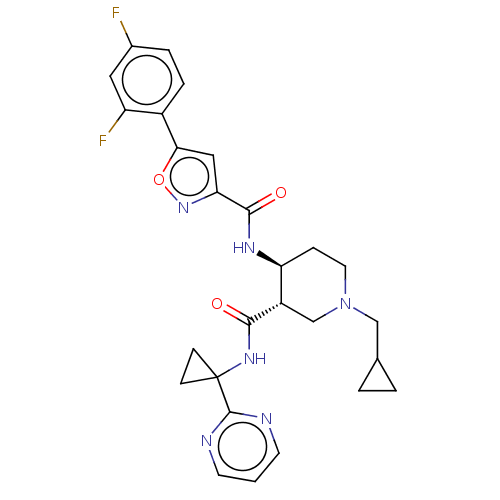 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to SNAP-tag fused human CXCR7 expressed in HEK293 cells by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555334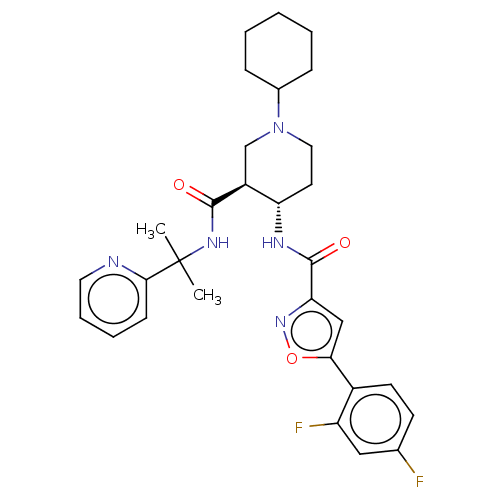 (CHEMBL4758472 | US11306078, Example 1.001b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555345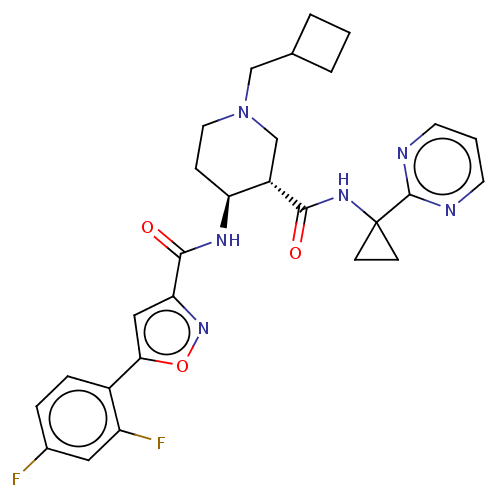 (CHEMBL4799528 | US11306078, Example 2.072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555336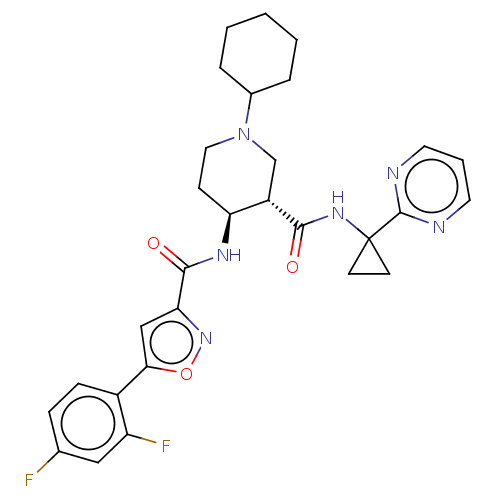 (CHEMBL4794973 | US11306078, Example 1.002b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.864 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.903 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.962 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.985 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.989 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409078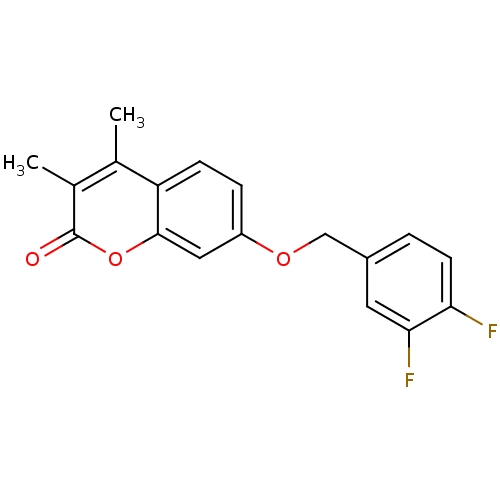 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555296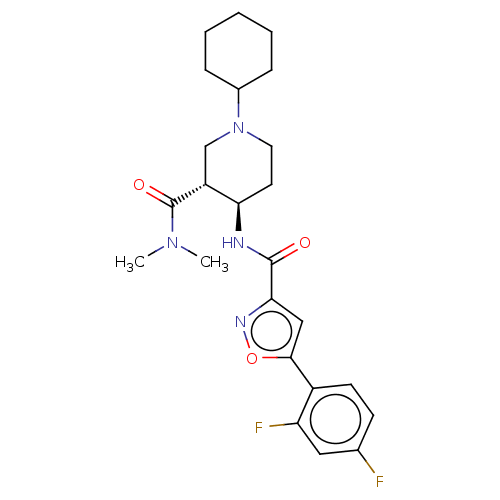 (CHEMBL4753893 | US11306078, Example 1.186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in CHO-K1 cells co-expressing beta-arrestin assessed as reduction in compound-1 induced respo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387199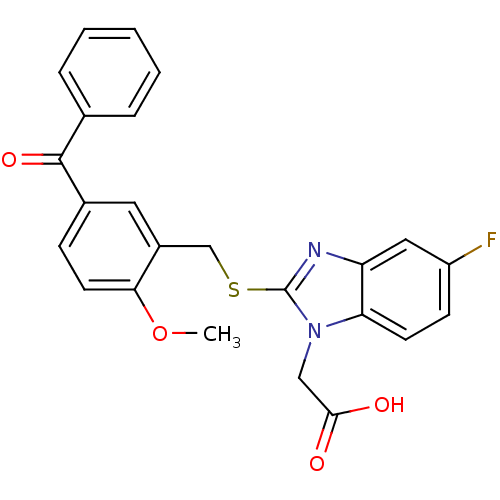 (CHEMBL2048197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555346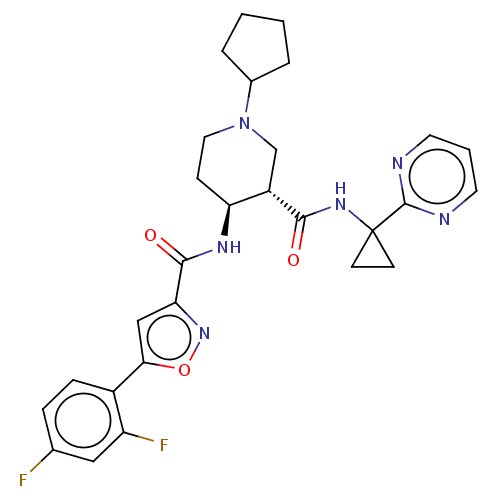 (CHEMBL4741307 | US11306078, Example 4.029) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555267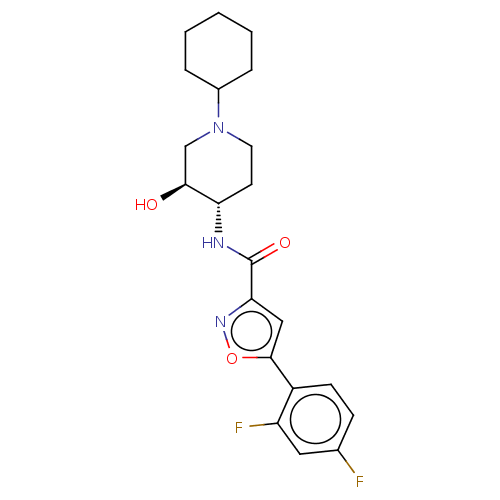 (CHEMBL4794569) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in CHO-K1 cells co-expressing beta-arrestin assessed as reduction in compound-1 induced respo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387205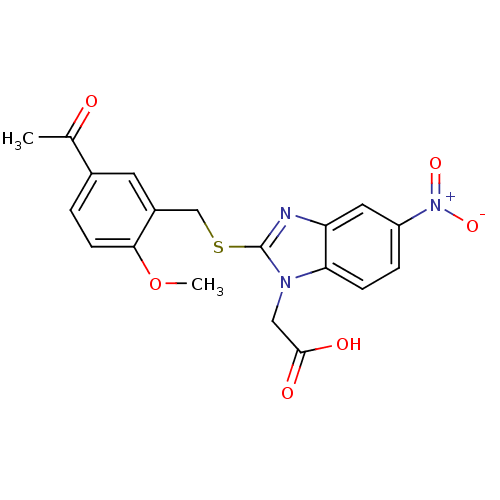 (CHEMBL2048189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387184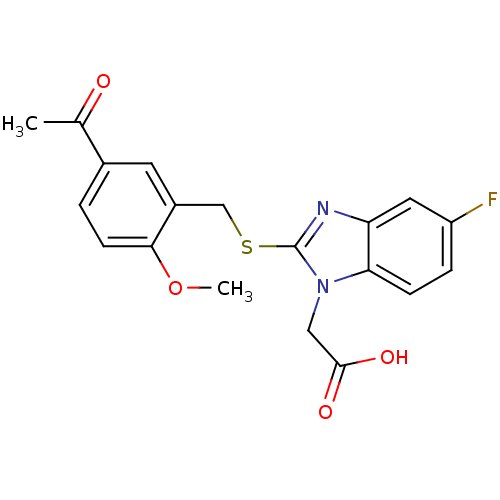 (CHEMBL2048187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555297 (CHEMBL4744654) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in CHO-K1 cells co-expressing beta-arrestin assessed as reduction in compound-1 induced respo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555261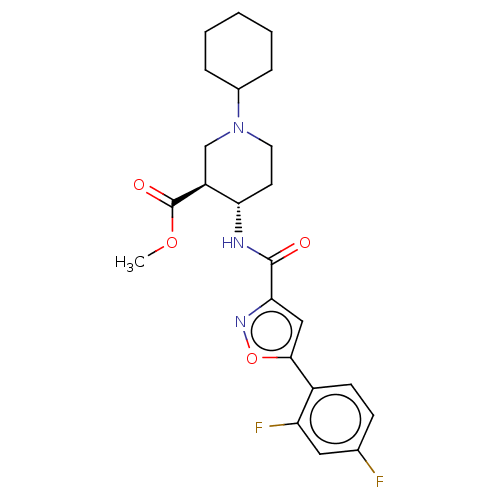 (CHEMBL4795114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in CHO-K1 cells co-expressing beta-arrestin assessed as reduction in compound-1 induced respo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387187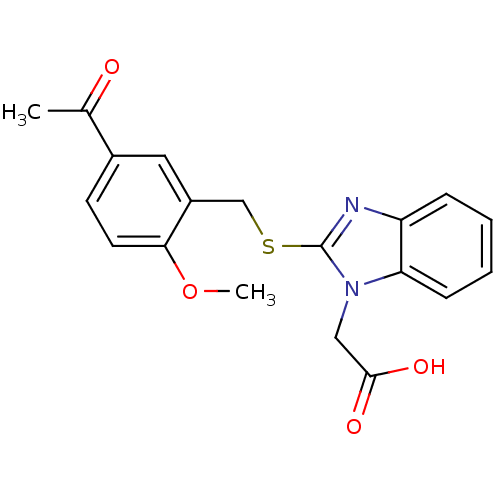 (CHEMBL2048183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells in presence of 0.5% human serum albumin | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Mus musculus) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse CXCR7 expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced response i... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Canis lupus familiaris) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at dog CXCR7 expressed in CHO-K1 cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced response incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409099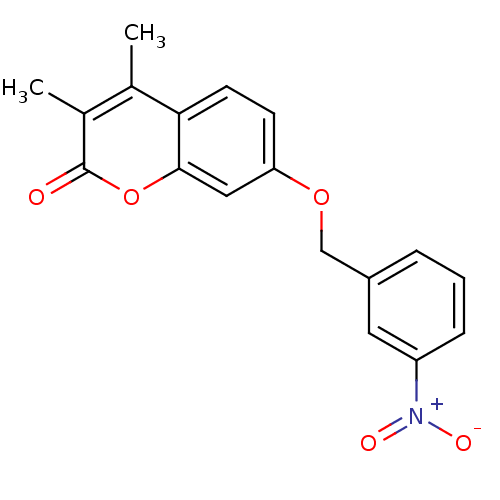 (CHEMBL356977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50282510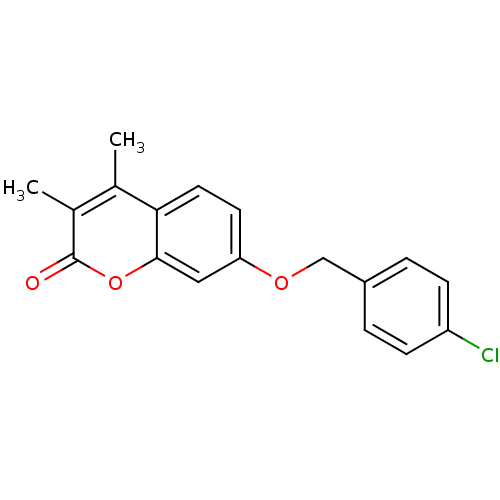 (7-(4-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555347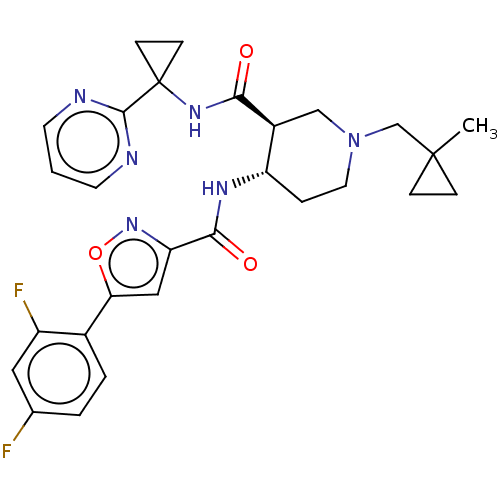 (CHEMBL4758009 | US11306078, Example 2.09) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555325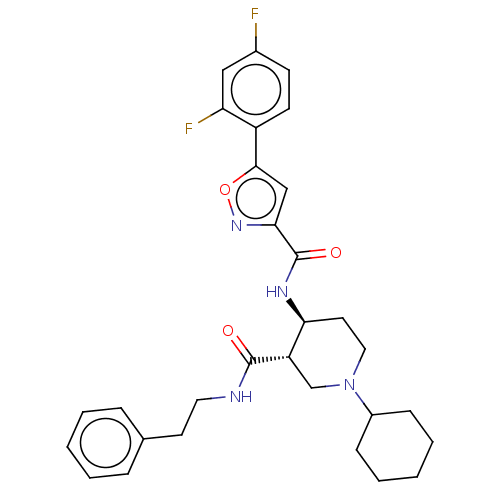 (CHEMBL4740027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Insurmountable antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409097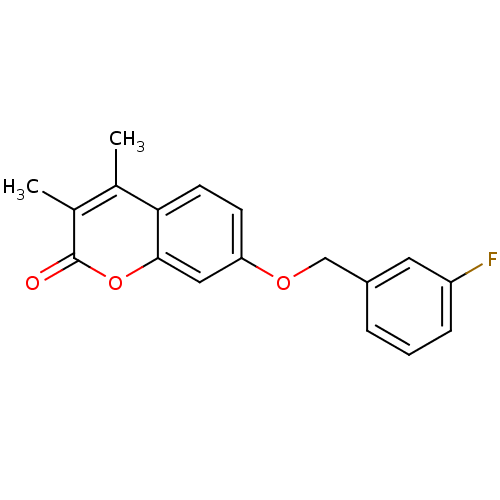 (CHEMBL108697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387205 (CHEMBL2048189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CRTh2 receptor expressed in HEK293 cells assessed as inhibition of PGD2-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387189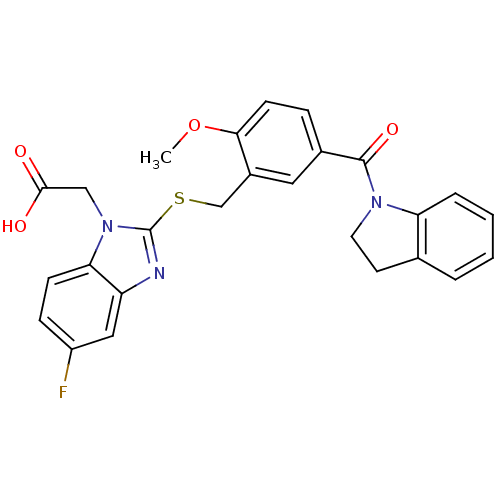 (CHEMBL2048204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387204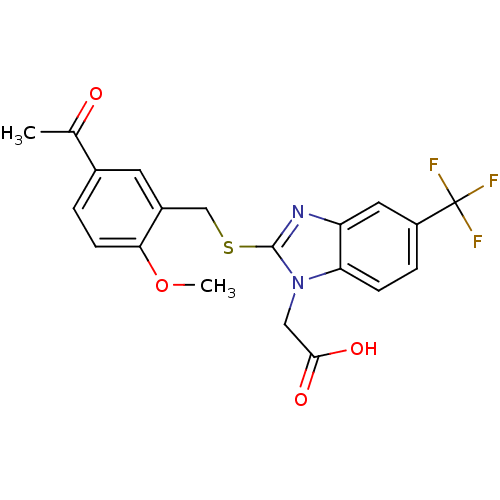 (CHEMBL2048190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50282502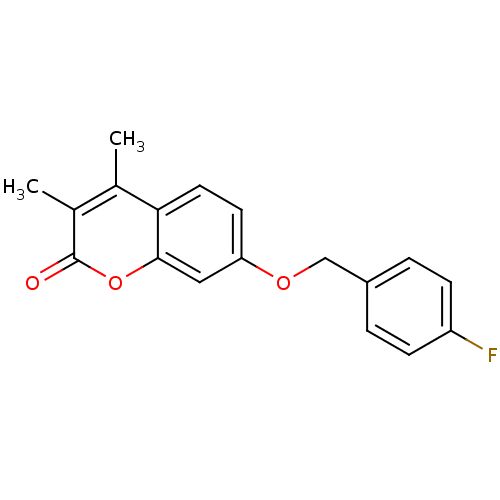 (7-(4-Fluoro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409109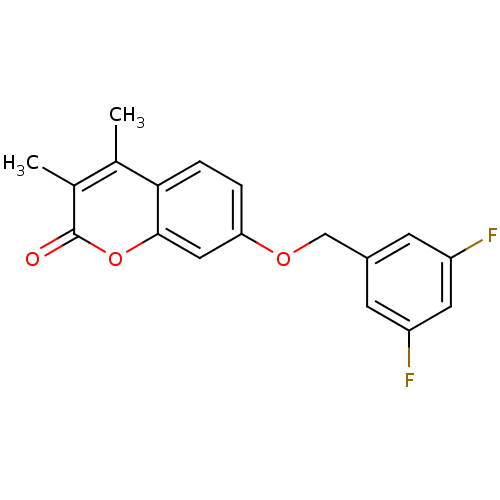 (CHEMBL108928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Rattus norvegicus) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat CXCR7 expressed in HEK293 cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced response incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50555343 (CHEMBL4782111 | US11306078, Example 4.032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01588 BindingDB Entry DOI: 10.7270/Q20P13P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50231957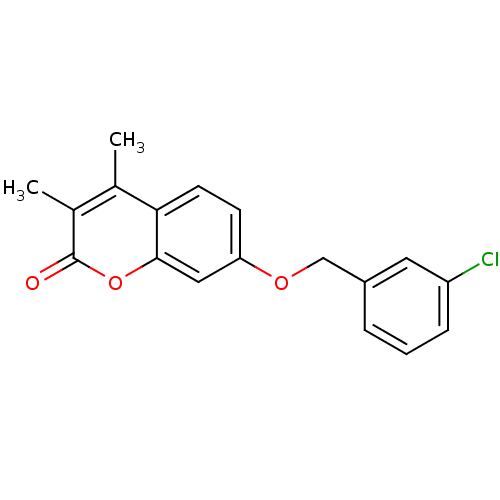 (7-(3-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409101 (CHEMBL142799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409092 (CHEMBL146222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409093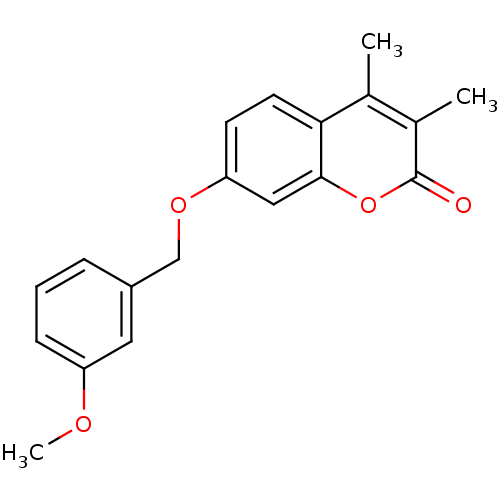 (CHEMBL111310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409084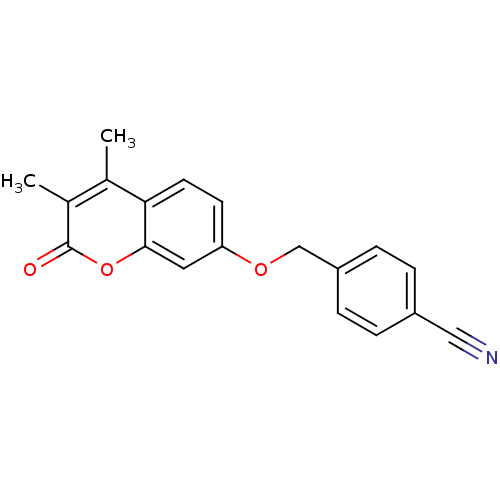 (CHEMBL145792) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387189 (CHEMBL2048204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CRTh2 receptor expressed in HEK293 cells assessed as inhibition of PGD2-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387199 (CHEMBL2048197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CRTh2 receptor expressed in HEK293 cells assessed as inhibition of PGD2-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387200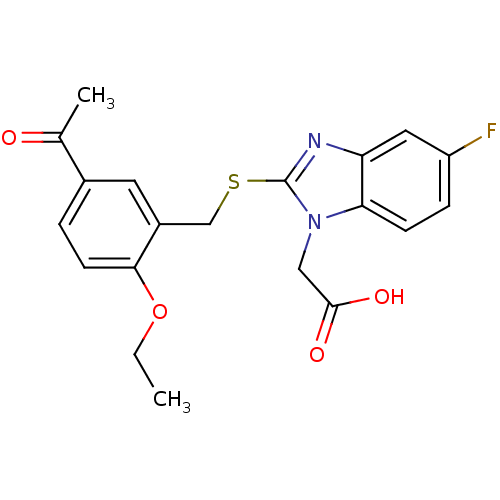 (CHEMBL2048194) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50387201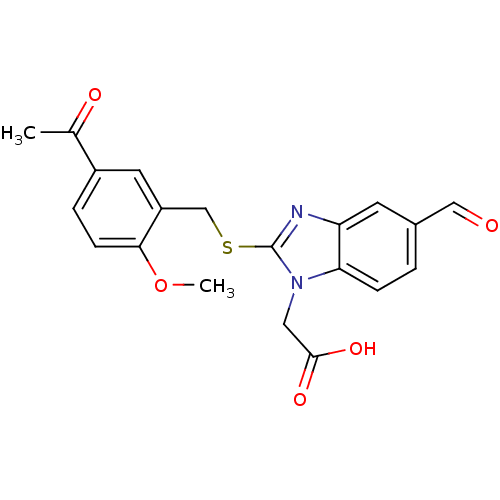 (CHEMBL2048193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from human CRTh2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 4660-4 (2012) Article DOI: 10.1016/j.bmcl.2012.05.087 BindingDB Entry DOI: 10.7270/Q2M32WTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 625 total ) | Next | Last >> |