

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304090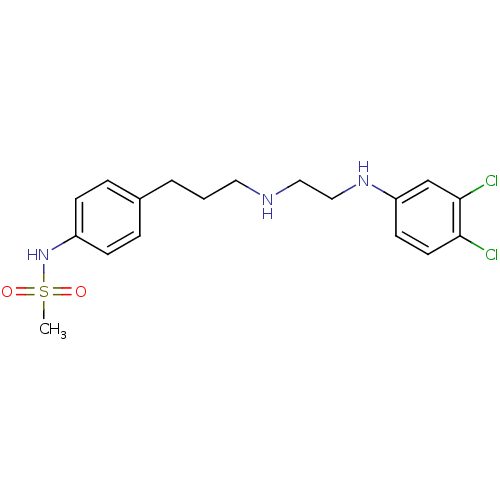 (CHEMBL594615 | N-(4-(3-(2-(3,4-Dichlorophenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304085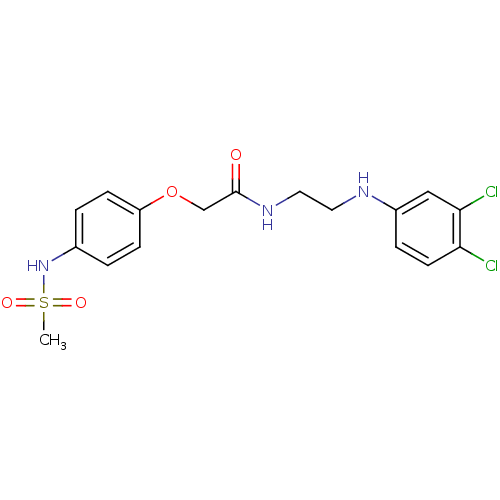 (CHEMBL607819 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304088 (CHEMBL596046 | N-(4-(2-(2-(3,4-Dichlorophenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304087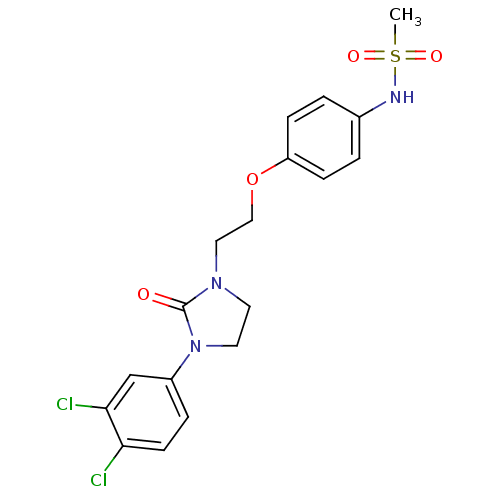 (CHEMBL596036 | N-(4-(2-(3-(3,4-Dichlorophenyl)-2-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168618 (US9079852, Table F, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304086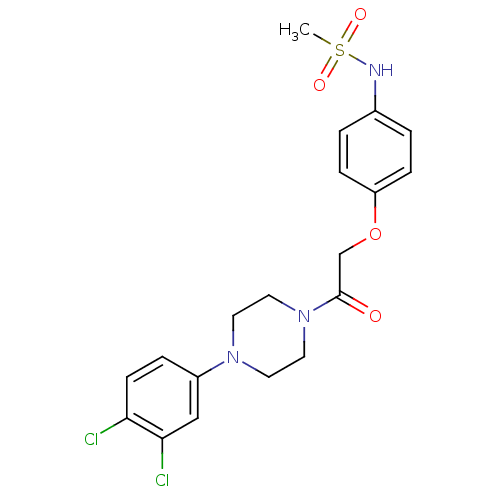 (CHEMBL594417 | N-(4-(2-(4-(3,4-Dichlorophenyl)pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168620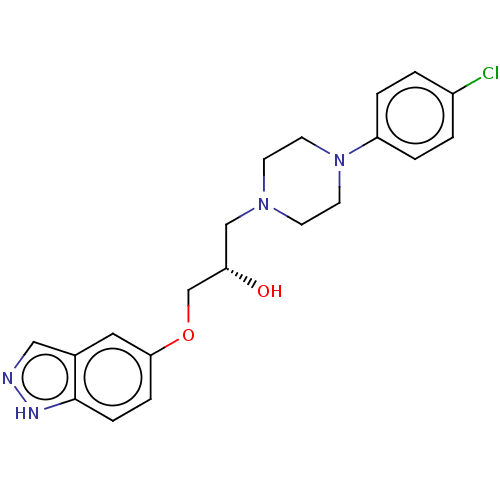 (US9079852, Table F, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168617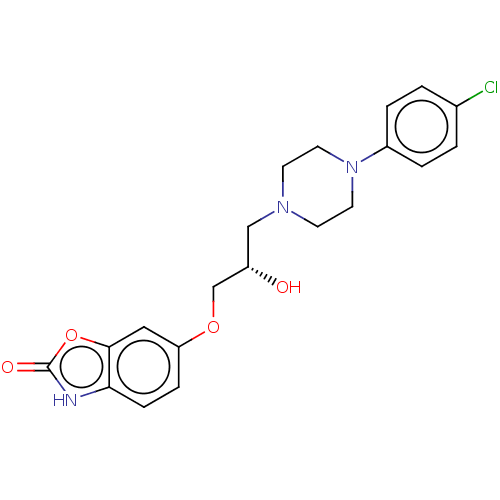 (US9079852, Table F, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304091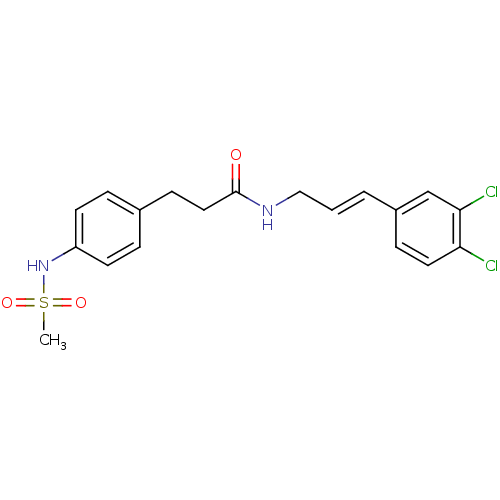 (CHEMBL609857 | N-(3,4-Dichlorocinnamyl)-3-(4-(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304089 (CHEMBL595318 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168626 (US9079852, Table F, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168625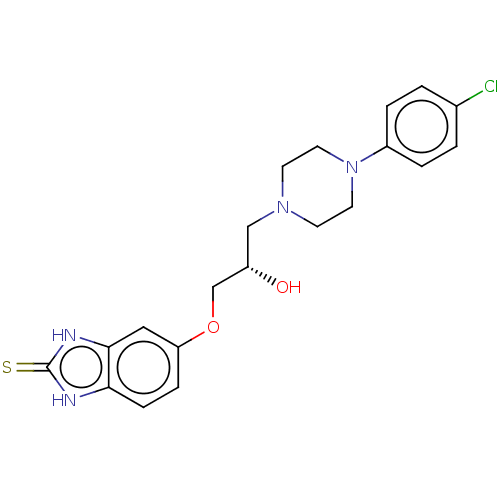 (US9079852, Table F, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168622 (US9079852, Table F, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168621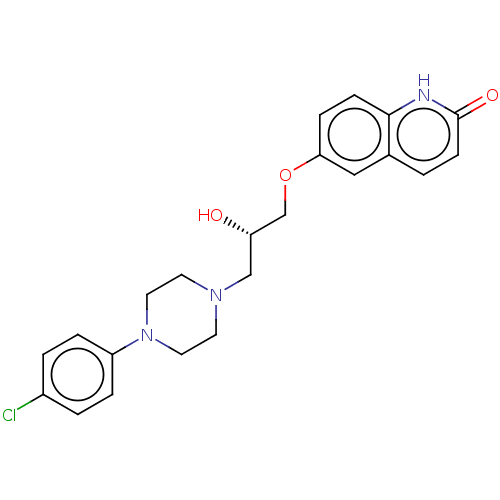 (US9079852, Table F, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168624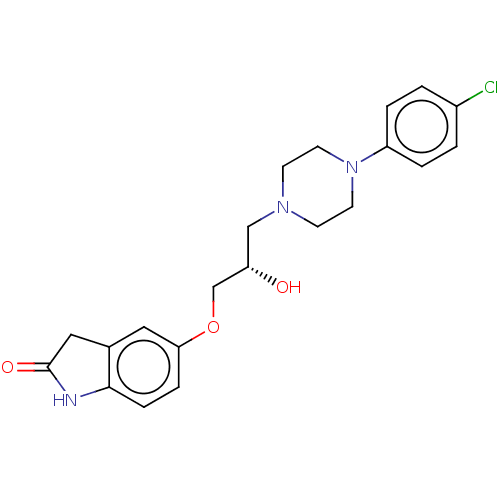 (US9079852, Table F, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168619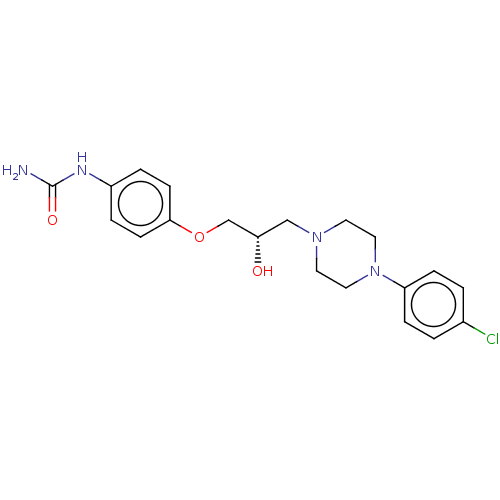 (US9079852, Table F, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168616 (US9079852, Table F, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50304085 (CHEMBL607819 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1/NR2B receptor expressed in frog oocytes assessed as inhibition of glutamate/glycine-induced current by two... | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50304085 (CHEMBL607819 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at wild type rat NR1/NR2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate/glycine-induced curren... | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168626 (US9079852, Table F, Compound 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168624 (US9079852, Table F, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168625 (US9079852, Table F, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168620 (US9079852, Table F, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168619 (US9079852, Table F, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168621 (US9079852, Table F, Compound 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168622 (US9079852, Table F, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168618 (US9079852, Table F, Compound 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168617 (US9079852, Table F, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168616 (US9079852, Table F, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324399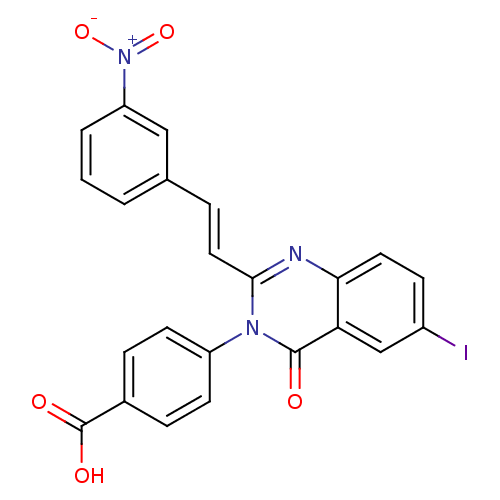 ((E)-4-(6-iodo-2-(3-nitrostyryl)-4-oxoquinazolin-3(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324405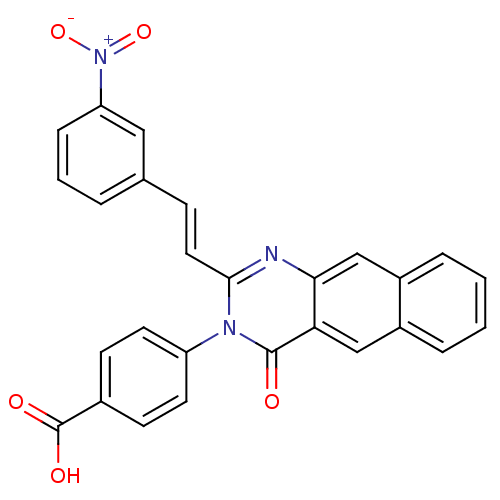 ((E)-4-(2-(3-Nitrostyryl)-4-oxobenzo[g]quinazolin-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324404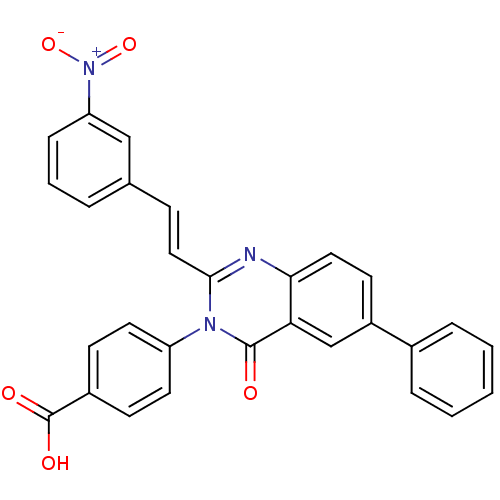 ((E)-4-(2-(3-Nitrostyryl)-4-oxo-6-phenylquinazolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324397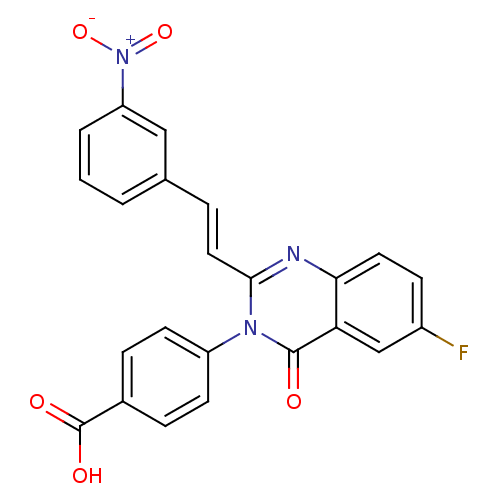 ((E)-4-(6-Fluoro-2-(3-nitrostyryl)-4-oxoquinazolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324398 ((E)-4-(6-Bromo-2-(3-nitrostyryl)-4-oxoquinazolin-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324428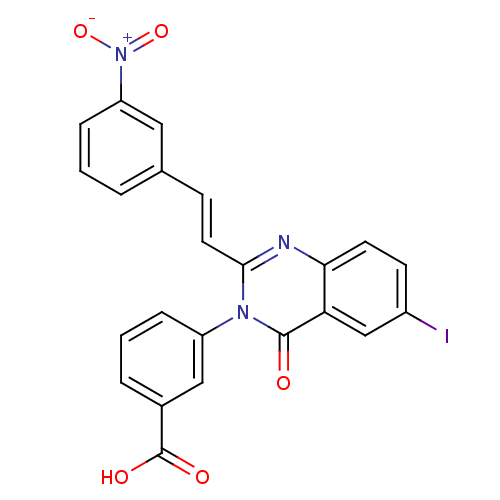 ((E)-3-(6-Iodo-2-(3-nitrostyryl)-4-oxoquinazolin-3(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324392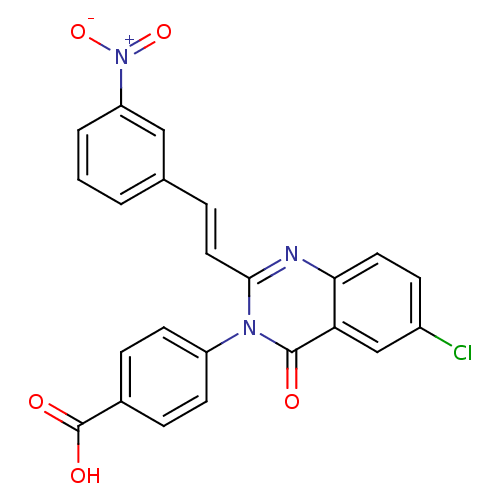 ((E)-4-(6-Chloro-2-(3-nitrostyryl)-4-oxoquinazolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324426 ((E)-4-(6-iodo-2-(4-nitrostyryl)-4-oxoquinazolin-3(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50324404 ((E)-4-(2-(3-Nitrostyryl)-4-oxo-6-phenylquinazolin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2B receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324445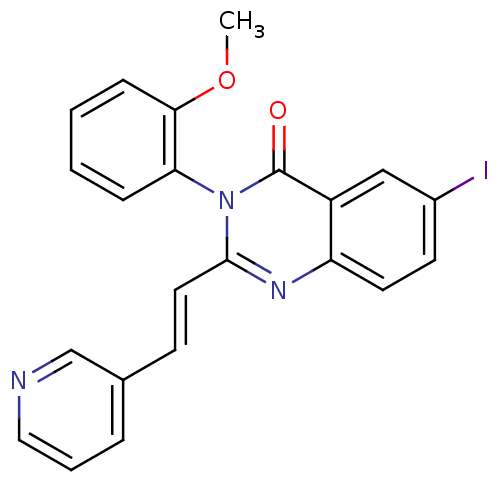 ((E)-6-iodo-3-(2-methoxyphenyl)-2-(2-(pyridin-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324409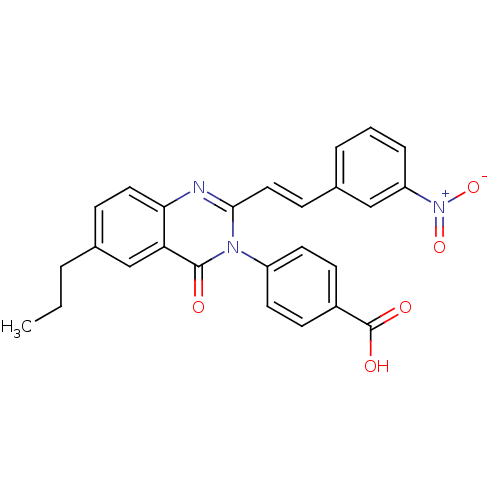 ((E)-4-(2-(3-Nitrostyryl)-4-oxo-6-propylquinazolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324411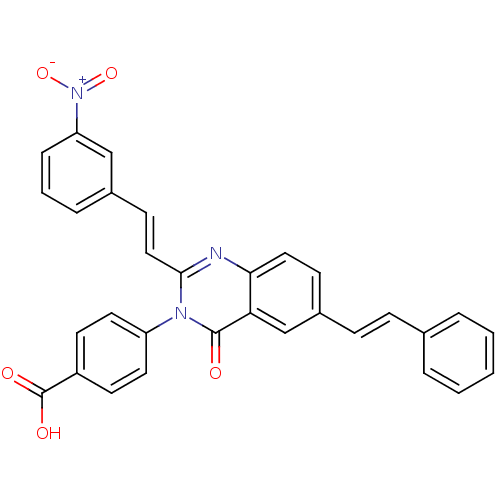 (4-(2-(3-Nitrostyryl)-4-oxo-6-styrylquinazolin-3(4H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50324405 ((E)-4-(2-(3-Nitrostyryl)-4-oxobenzo[g]quinazolin-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant GLuR1 expressed in Xenopus oocytes assessed as inhibition of glutamate-induced current by two-electrode voltag... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324473 ((E)-3-(2-methoxyphenyl)-2-(2-nitrostyryl)quinazoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM50324473 ((E)-3-(2-methoxyphenyl)-2-(2-nitrostyryl)quinazoli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2A receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324410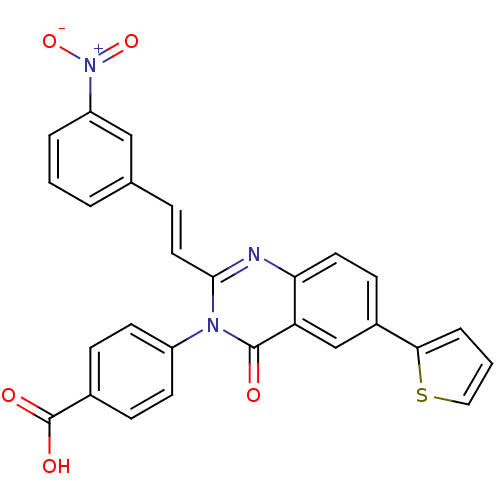 ((E)-4-(2-(3-nitrostyryl)-4-oxo-6-(thiophen-2-yl)qu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50324463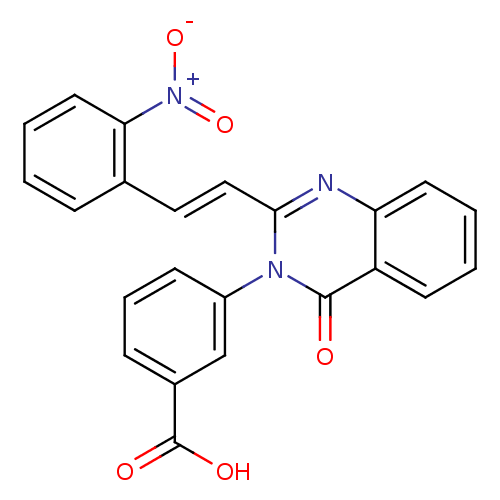 ((E)-3-(2-(2-nitrostyryl)-4-oxoquinazolin-3(4H)-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant GLuR1 expressed in Xenopus oocytes assessed as inhibition of glutamate-induced current by two-electrode voltag... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50324404 ((E)-4-(2-(3-Nitrostyryl)-4-oxo-6-phenylquinazolin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant GLuR1 expressed in Xenopus oocytes assessed as inhibition of glutamate-induced current by two-electrode voltag... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50324405 ((E)-4-(2-(3-Nitrostyryl)-4-oxobenzo[g]quinazolin-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2B receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM50324400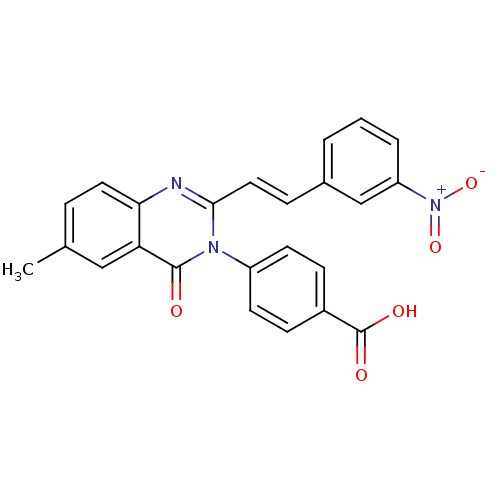 ((E)-4-(6-Methyl-2-(3-nitrostyryl)-4-oxoquinazolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant NR1-NR2C receptor expressed in Xenopus oocytes at holding potential of -40mV by two-electrode voltage-clamp el... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50324461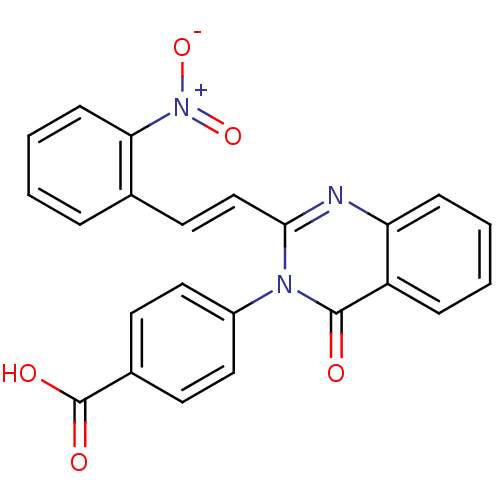 ((E)-4-(2-(2-nitrostyryl)-4-oxoquinazolin-3(4H)-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant GLuR1 expressed in Xenopus oocytes assessed as inhibition of glutamate-induced current by two-electrode voltag... | J Med Chem 53: 5476-90 (2010) Article DOI: 10.1021/jm100027p BindingDB Entry DOI: 10.7270/Q2DR2VQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 405 total ) | Next | Last >> |