 Found 2794 hits with Last Name = 'neagu' and Initial = 'c'
Found 2794 hits with Last Name = 'neagu' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50463725

(CHEMBL4242598)Show SMILES [H][C@]12CN(c3ccc(C(N)=O)c(Oc4ccc(Oc5ccccc5)cc4)n3)[C@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C26H24N4O4/c1-2-24(31)30-16-17-14-18(30)15-29(17)23-13-12-22(25(27)32)26(28-23)34-21-10-8-20(9-11-21)33-19-6-4-3-5-7-19/h2-13,17-18H,1,14-16H2,(H2,27,32)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of POP |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAP |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 3
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP3 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182699

(US9145392, 400)Show SMILES CCc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CC1CN(C)C1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C26H34FN7/c1-4-21-24(28)29-16-30-26(21)33-9-7-19(8-10-33)25-31-23(20-5-6-22(27)17(2)11-20)15-34(25)14-18-12-32(3)13-18/h5-6,11,15-16,18-19H,4,7-10,12-14H2,1-3H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466323
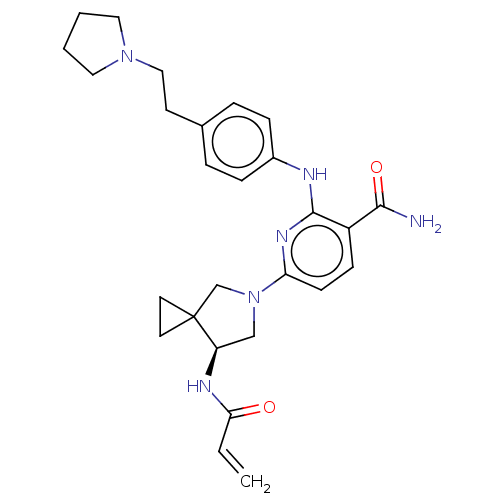
(CHEMBL4279353)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(CCN2CCCC2)cc1)N1C[C@@H](NC(=O)C=C)C2(CC2)C1 |r| Show InChI InChI=1S/C27H34N6O2/c1-2-24(34)30-22-17-33(18-27(22)12-13-27)23-10-9-21(25(28)35)26(31-23)29-20-7-5-19(6-8-20)11-16-32-14-3-4-15-32/h2,5-10,22H,1,3-4,11-18H2,(H2,28,35)(H,29,31)(H,30,34)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182489

(US9145392, 190)Show SMILES Cc1cc(ccc1F)-c1cn(CCN2CCC2)c(n1)C1CCN(CC1)c1ncnc(N)c1Br Show InChI InChI=1S/C24H29BrFN7/c1-16-13-18(3-4-19(16)26)20-14-33(12-11-31-7-2-8-31)23(30-20)17-5-9-32(10-6-17)24-21(25)22(27)28-15-29-24/h3-4,13-15,17H,2,5-12H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466316
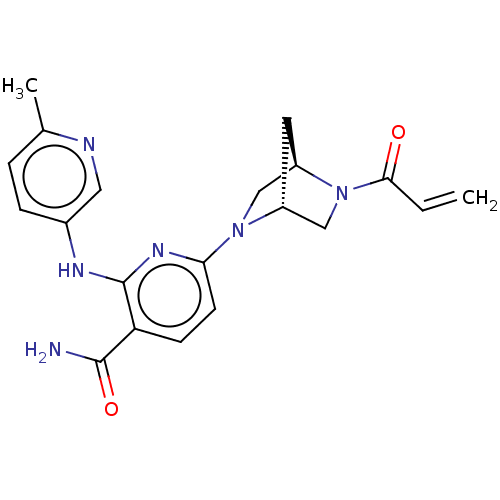
(CHEMBL4276669)Show SMILES [H][C@@]12CN(c3ccc(C(N)=O)c(Nc4ccc(C)nc4)n3)[C@@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C20H22N6O2/c1-3-18(27)26-11-14-8-15(26)10-25(14)17-7-6-16(19(21)28)20(24-17)23-13-5-4-12(2)22-9-13/h3-7,9,14-15H,1,8,10-11H2,2H3,(H2,21,28)(H,23,24)/t14-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182489

(US9145392, 190)Show SMILES Cc1cc(ccc1F)-c1cn(CCN2CCC2)c(n1)C1CCN(CC1)c1ncnc(N)c1Br Show InChI InChI=1S/C24H29BrFN7/c1-16-13-18(3-4-19(16)26)20-14-33(12-11-31-7-2-8-31)23(30-20)17-5-9-32(10-6-17)24-21(25)22(27)28-15-29-24/h3-4,13-15,17H,2,5-12H2,1H3,(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182480

(US9145392, 181)Show SMILES CC(C)c1cc(ccn1)-c1cn(CCN2CCC2)c(n1)C1CCN(CC1)c1ncnc(N)c1Br Show InChI InChI=1S/C25H33BrN8/c1-17(2)20-14-19(4-7-28-20)21-15-34(13-12-32-8-3-9-32)24(31-21)18-5-10-33(11-6-18)25-22(26)23(27)29-16-30-25/h4,7,14-18H,3,5-6,8-13H2,1-2H3,(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182480

(US9145392, 181)Show SMILES CC(C)c1cc(ccn1)-c1cn(CCN2CCC2)c(n1)C1CCN(CC1)c1ncnc(N)c1Br Show InChI InChI=1S/C25H33BrN8/c1-17(2)20-14-19(4-7-28-20)21-15-34(13-12-32-8-3-9-32)24(31-21)18-5-10-33(11-6-18)25-22(26)23(27)29-16-30-25/h4,7,14-18H,3,5-6,8-13H2,1-2H3,(H2,27,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182671
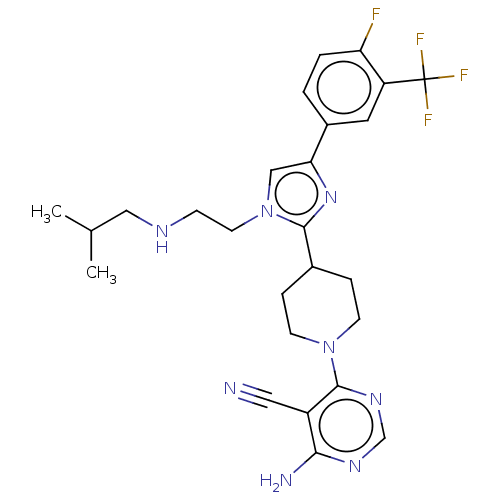
(US9145392, 372)Show SMILES CC(C)CNCCn1cc(nc1C1CCN(CC1)c1ncnc(N)c1C#N)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C26H30F4N8/c1-16(2)13-33-7-10-38-14-22(18-3-4-21(27)20(11-18)26(28,29)30)36-24(38)17-5-8-37(9-6-17)25-19(12-31)23(32)34-15-35-25/h3-4,11,14-17,33H,5-10,13H2,1-2H3,(H2,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182551
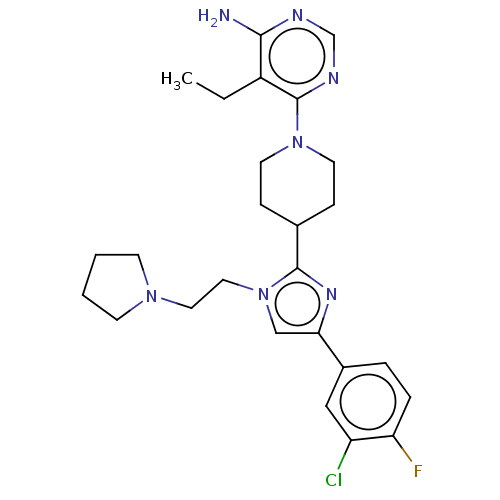
(US9145392, 252)Show SMILES CCc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CCN1CCCC1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C26H33ClFN7/c1-2-20-24(29)30-17-31-26(20)34-11-7-18(8-12-34)25-32-23(19-5-6-22(28)21(27)15-19)16-35(25)14-13-33-9-3-4-10-33/h5-6,15-18H,2-4,7-14H2,1H3,(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571038
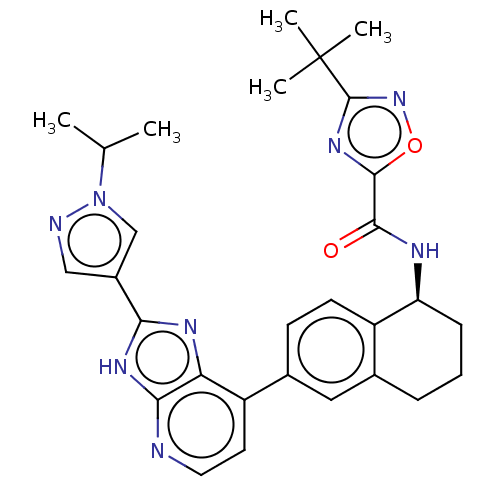
(CHEMBL4868813)Show SMILES CC(C)n1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182483

(US9145392, 184)Show SMILES COc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CC1CNC1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C24H30FN7O/c1-15-9-18(3-4-19(15)25)20-13-32(12-16-10-27-11-16)23(30-20)17-5-7-31(8-6-17)24-21(33-2)22(26)28-14-29-24/h3-4,9,13-14,16-17,27H,5-8,10-12H2,1-2H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182699

(US9145392, 400)Show SMILES CCc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CC1CN(C)C1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C26H34FN7/c1-4-21-24(28)29-16-30-26(21)33-9-7-19(8-10-33)25-31-23(20-5-6-22(27)17(2)11-20)15-34(25)14-18-12-32(3)13-18/h5-6,11,15-16,18-19H,4,7-10,12-14H2,1-3H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466325
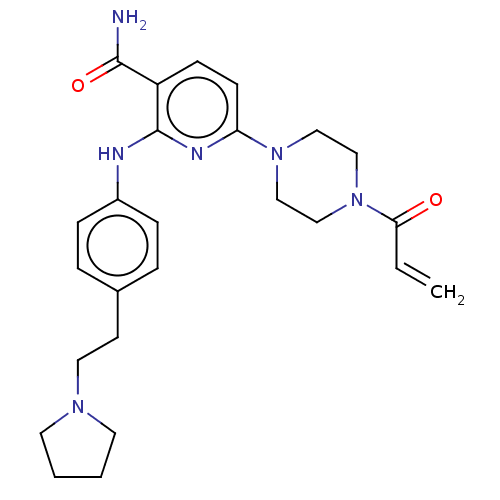
(CHEMBL4279430)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(CCN2CCCC2)cc1)N1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C25H32N6O2/c1-2-23(32)31-17-15-30(16-18-31)22-10-9-21(24(26)33)25(28-22)27-20-7-5-19(6-8-20)11-14-29-12-3-4-13-29/h2,5-10H,1,3-4,11-18H2,(H2,26,33)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514956
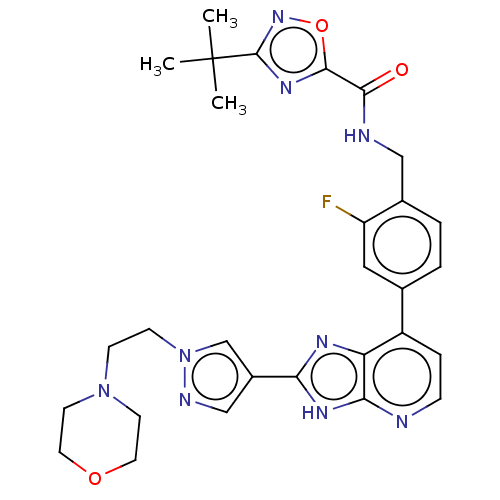
(US11098041, Example 187)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(CCN2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514891
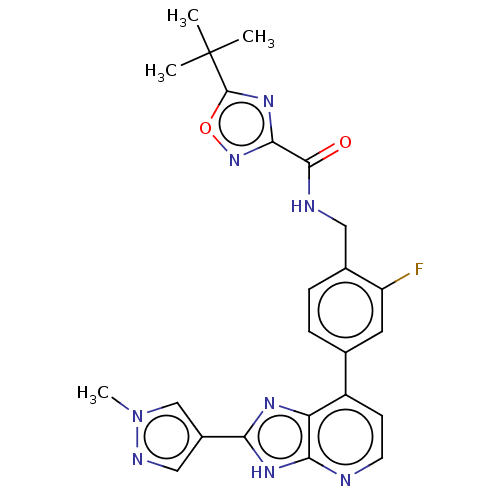
(US11098041, Example 116)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2noc(n2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182673

(US9145392, 374)Show SMILES CNCCn1cc(nc1C1CCN(CC1)c1ncnc(N)c1C#N)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C23H24F4N8/c1-30-6-9-35-12-19(15-2-3-18(24)17(10-15)23(25,26)27)33-21(35)14-4-7-34(8-5-14)22-16(11-28)20(29)31-13-32-22/h2-3,10,12-14,30H,4-9H2,1H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182551

(US9145392, 252)Show SMILES CCc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CCN1CCCC1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C26H33ClFN7/c1-2-20-24(29)30-17-31-26(20)34-11-7-18(8-12-34)25-32-23(19-5-6-22(28)21(27)15-19)16-35(25)14-13-33-9-3-4-10-33/h5-6,15-18H,2-4,7-14H2,1H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514887
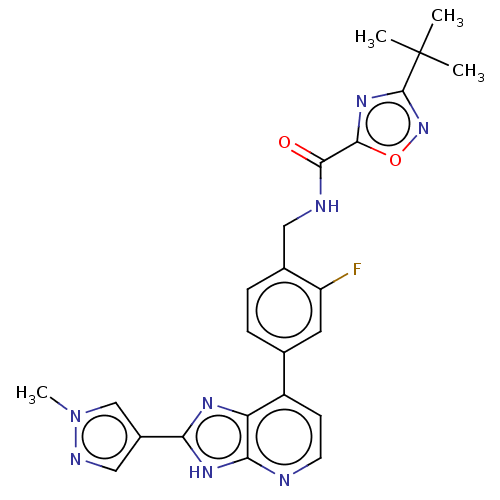
(US11098041, Example 112)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571039
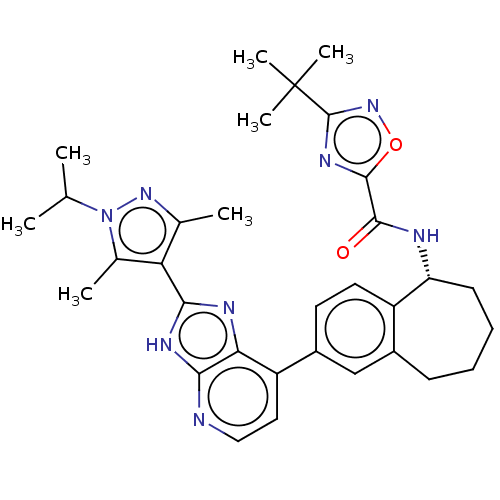
(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514935
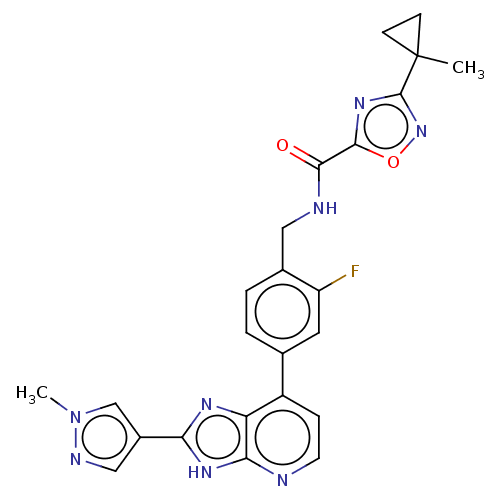
(US11098041, Example 165)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C2(C)CC2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182473
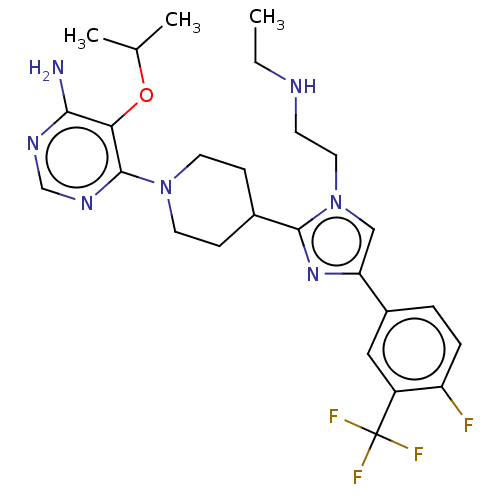
(US9145392, 174)Show SMILES CCNCCn1cc(nc1C1CCN(CC1)c1ncnc(N)c1OC(C)C)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C26H33F4N7O/c1-4-32-9-12-37-14-21(18-5-6-20(27)19(13-18)26(28,29)30)35-24(37)17-7-10-36(11-8-17)25-22(38-16(2)3)23(31)33-15-34-25/h5-6,13-17,32H,4,7-12H2,1-3H3,(H2,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466328
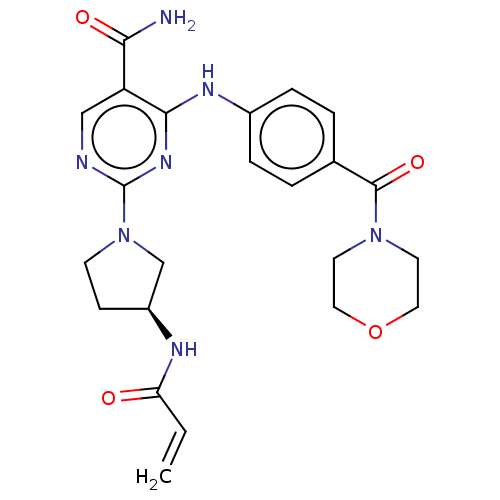
(CHEMBL4290540)Show SMILES NC(=O)c1cnc(nc1Nc1ccc(cc1)C(=O)N1CCOCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C23H27N7O4/c1-2-19(31)26-17-7-8-30(14-17)23-25-13-18(20(24)32)21(28-23)27-16-5-3-15(4-6-16)22(33)29-9-11-34-12-10-29/h2-6,13,17H,1,7-12,14H2,(H2,24,32)(H,26,31)(H,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571033
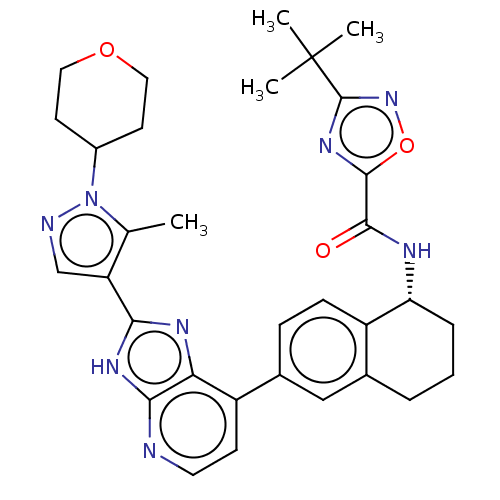
(CHEMBL4876326)Show SMILES Cc1c(cnn1C1CCOCC1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514915

(US11098041, Example 144)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182624
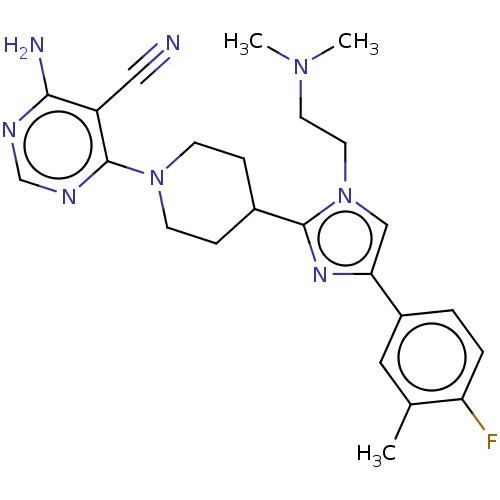
(US9145392, 325)Show SMILES CN(C)CCn1cc(nc1C1CCN(CC1)c1ncnc(N)c1C#N)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C24H29FN8/c1-16-12-18(4-5-20(16)25)21-14-33(11-10-31(2)3)23(30-21)17-6-8-32(9-7-17)24-19(13-26)22(27)28-15-29-24/h4-5,12,14-15,17H,6-11H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182535

(US9145392, 236)Show SMILES Nc1ncnc(N2CCC(CC2)c2nc(cn2CCN2CCCC2)-c2cccc(F)c2)c1Cl Show InChI InChI=1S/C24H29ClFN7/c25-21-22(27)28-16-29-24(21)32-10-6-17(7-11-32)23-30-20(18-4-3-5-19(26)14-18)15-33(23)13-12-31-8-1-2-9-31/h3-5,14-17H,1-2,6-13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182425
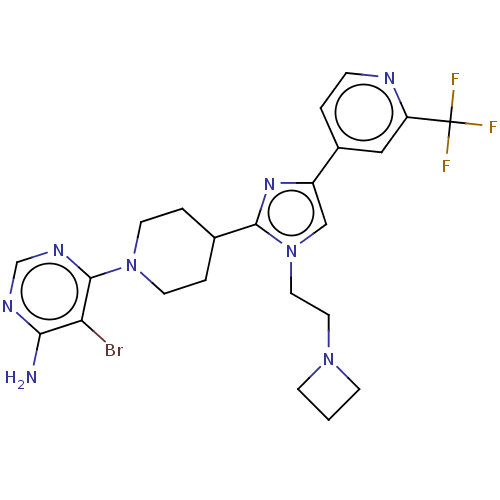
(US9145392, 126)Show SMILES Nc1ncnc(N2CCC(CC2)c2nc(cn2CCN2CCC2)-c2ccnc(c2)C(F)(F)F)c1Br Show InChI InChI=1S/C23H26BrF3N8/c24-19-20(28)30-14-31-22(19)34-8-3-15(4-9-34)21-32-17(13-35(21)11-10-33-6-1-7-33)16-2-5-29-18(12-16)23(25,26)27/h2,5,12-15H,1,3-4,6-11H2,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514904
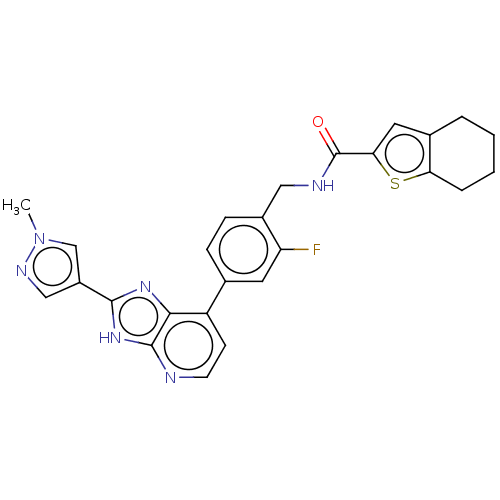
(US11098041, Example 132)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc3CCCCc3s2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182473

(US9145392, 174)Show SMILES CCNCCn1cc(nc1C1CCN(CC1)c1ncnc(N)c1OC(C)C)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C26H33F4N7O/c1-4-32-9-12-37-14-21(18-5-6-20(27)19(13-18)26(28,29)30)35-24(37)17-7-10-36(11-8-17)25-22(38-16(2)3)23(31)33-15-34-25/h5-6,13-17,32H,4,7-12H2,1-3H3,(H2,31,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182517

(US9145392, 218)Show SMILES Nc1ncnc(N2CCC(CC2)c2nc(cn2CCN2CCC2)-c2ccc(F)c(c2)C(F)(F)F)c1Cl Show InChI InChI=1S/C24H26ClF4N7/c25-20-21(30)31-14-32-23(20)35-8-4-15(5-9-35)22-33-19(13-36(22)11-10-34-6-1-7-34)16-2-3-18(26)17(12-16)24(27,28)29/h2-3,12-15H,1,4-11H2,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466206
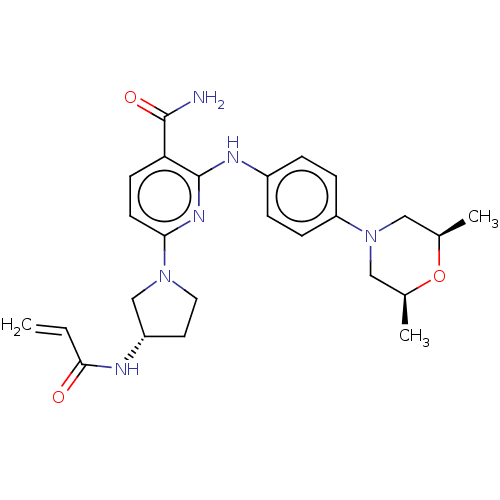
(CHEMBL4281335)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C25H32N6O3/c1-4-23(32)27-19-11-12-30(15-19)22-10-9-21(24(26)33)25(29-22)28-18-5-7-20(8-6-18)31-13-16(2)34-17(3)14-31/h4-10,16-17,19H,1,11-15H2,2-3H3,(H2,26,33)(H,27,32)(H,28,29)/t16-,17+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182470
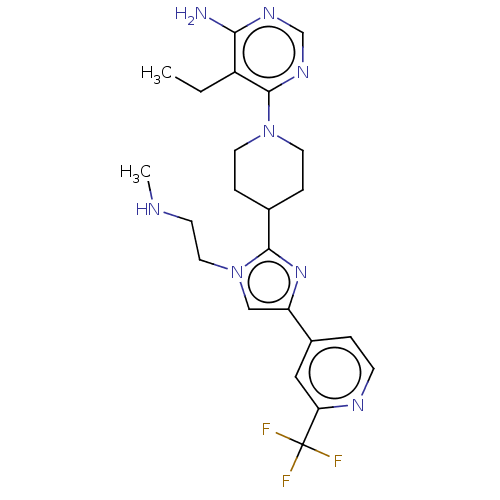
(US9145392, 171)Show SMILES CCc1c(N)ncnc1N1CCC(CC1)c1nc(cn1CCNC)-c1ccnc(c1)C(F)(F)F Show InChI InChI=1S/C23H29F3N8/c1-3-17-20(27)30-14-31-22(17)33-9-5-15(6-10-33)21-32-18(13-34(21)11-8-28-2)16-4-7-29-19(12-16)23(24,25)26/h4,7,12-15,28H,3,5-6,8-11H2,1-2H3,(H2,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182305

(US9145392, 6)Show SMILES Cn1cc(nc1C1CCN(CC1)c1ncnc(N)c1C=O)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C21H20F4N6O/c1-30-9-17(13-2-3-16(22)15(8-13)21(23,24)25)29-19(30)12-4-6-31(7-5-12)20-14(10-32)18(26)27-11-28-20/h2-3,8-12H,4-7H2,1H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571037
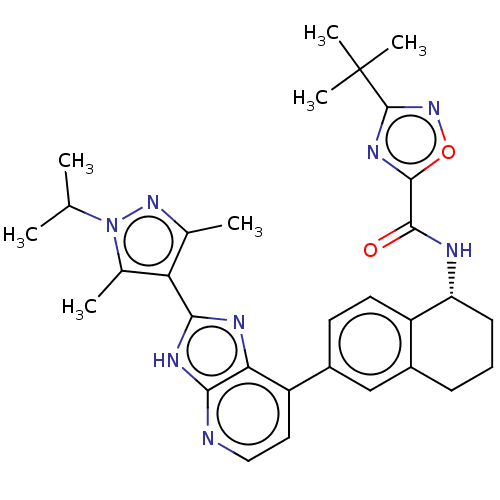
(CHEMBL4862532)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM182586
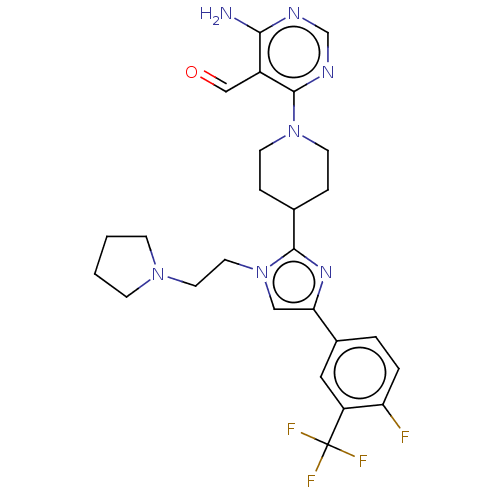
(US9145392, 287)Show SMILES Nc1ncnc(N2CCC(CC2)c2nc(cn2CCN2CCCC2)-c2ccc(F)c(c2)C(F)(F)F)c1C=O Show InChI InChI=1S/C26H29F4N7O/c27-21-4-3-18(13-20(21)26(28,29)30)22-14-37(12-11-35-7-1-2-8-35)24(34-22)17-5-9-36(10-6-17)25-19(15-38)23(31)32-16-33-25/h3-4,13-17H,1-2,5-12H2,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
P70S6K inhibitor compounds are diluted and plated in 96 well plates. A reaction mixture including the following components is then added to the compo... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50463725

(CHEMBL4242598)Show SMILES [H][C@]12CN(c3ccc(C(N)=O)c(Oc4ccc(Oc5ccccc5)cc4)n3)[C@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C26H24N4O4/c1-2-24(31)30-16-17-14-18(30)15-29(17)23-13-12-22(25(27)32)26(28-23)34-21-10-8-20(9-11-21)33-19-6-4-3-5-7-19/h2-13,17-18H,1,14-16H2,(H2,27,32)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571029
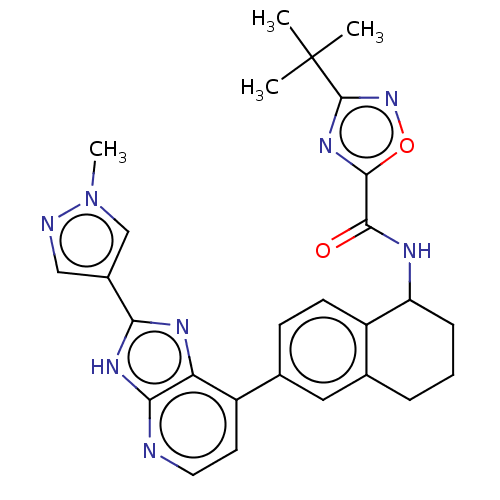
(CHEMBL4855860)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2C(CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514897
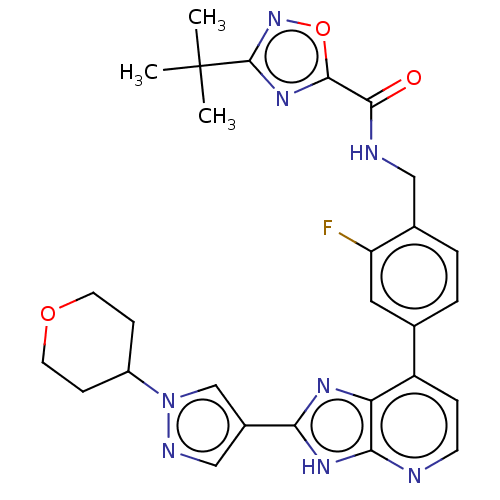
(US11098041, Example 124)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(c1)C1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM182688

(US9145392, 389)Show SMILES Cc1cc(ccc1F)-c1cn(CCNC2CCCC2)c(n1)C1CCN(CC1)c1ncnc(N)c1C#N Show InChI InChI=1S/C27H33FN8/c1-18-14-20(6-7-23(18)28)24-16-36(13-10-31-21-4-2-3-5-21)26(34-24)19-8-11-35(12-9-19)27-22(15-29)25(30)32-17-33-27/h6-7,14,16-17,19,21,31H,2-5,8-13H2,1H3,(H2,30,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH
US Patent
| Assay Description
A TTP Mosquito liquid handling instrument is used to place 125n1 of the appropriate concentration of inhibitor in 100% DMSO (for a dose response curv... |
US Patent US9145392 (2015)
BindingDB Entry DOI: 10.7270/Q2NZ86D2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data