 Found 572 hits with Last Name = 'santos' and Initial = 'c'
Found 572 hits with Last Name = 'santos' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50385972
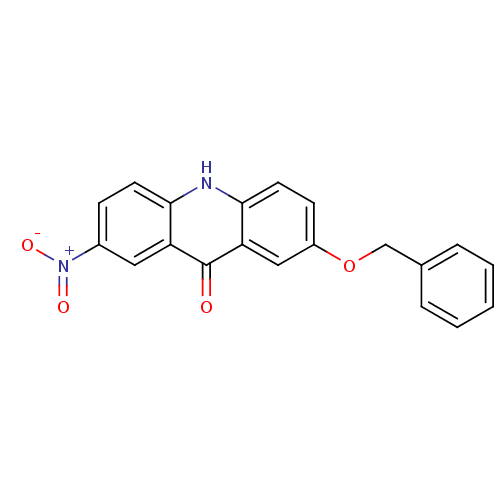
(CHEMBL2042470)Show SMILES [O-][N+](=O)c1ccc2[nH]c3ccc(OCc4ccccc4)cc3c(=O)c2c1 Show InChI InChI=1S/C20H14N2O4/c23-20-16-10-14(22(24)25)6-8-18(16)21-19-9-7-15(11-17(19)20)26-12-13-4-2-1-3-5-13/h1-11H,12H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50385972

(CHEMBL2042470)Show SMILES [O-][N+](=O)c1ccc2[nH]c3ccc(OCc4ccccc4)cc3c(=O)c2c1 Show InChI InChI=1S/C20H14N2O4/c23-20-16-10-14(22(24)25)6-8-18(16)21-19-9-7-15(11-17(19)20)26-12-13-4-2-1-3-5-13/h1-11H,12H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50438272
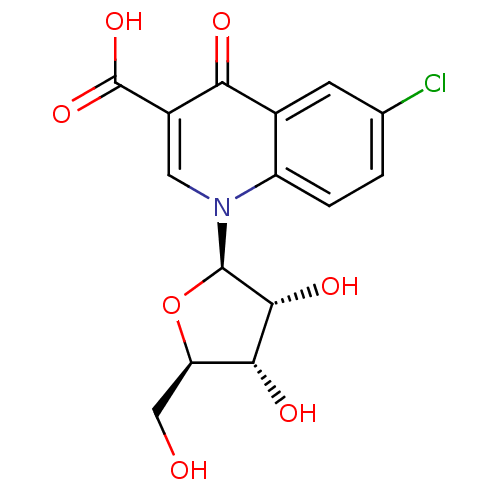
(CHEMBL560455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C(O)=O)c(=O)c2cc(Cl)ccc12 |r| Show InChI InChI=1S/C15H14ClNO7/c16-6-1-2-9-7(3-6)11(19)8(15(22)23)4-17(9)14-13(21)12(20)10(5-18)24-14/h1-4,10,12-14,18,20-21H,5H2,(H,22,23)/t10-,12-,13-,14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Bioorg Med Chem Lett 22: 5055-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.020
BindingDB Entry DOI: 10.7270/Q2VX0KCN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291573
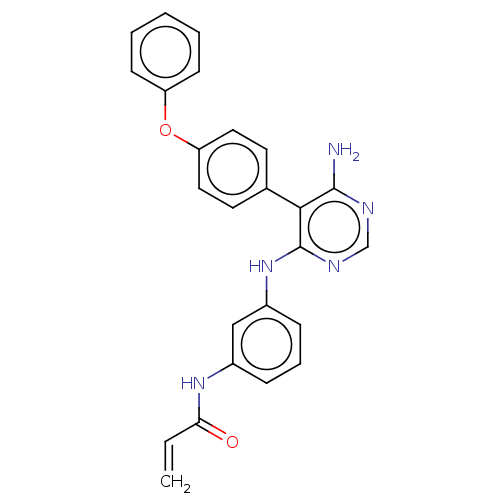
(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291413

(1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC2CC3(C2)CN(C3)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-21(31)30-14-25(15-30)12-18(13-25)29-24-22(23(26)27-16-28-24)17-8-10-20(11-9-17)32-19-6-4-3-5-7-19/h2-11,16,18H,1,12-15H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50385984
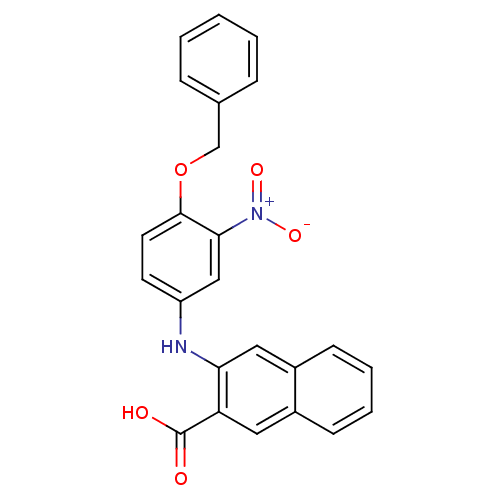
(CHEMBL2042454)Show SMILES OC(=O)c1cc2ccccc2cc1Nc1ccc(OCc2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H18N2O5/c27-24(28)20-12-17-8-4-5-9-18(17)13-21(20)25-19-10-11-23(22(14-19)26(29)30)31-15-16-6-2-1-3-7-16/h1-14,25H,15H2,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50385977
(CHEMBL2042446)Show InChI InChI=1S/C19H19NO/c1-2-3-6-14-9-11-15(12-10-14)18-13-19(21)16-7-4-5-8-17(16)20-18/h4-5,7-13H,2-3,6H2,1H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot anal... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50385966

(CHEMBL2042465)Show InChI InChI=1S/C20H15NO2/c22-20-16-8-4-5-9-18(16)21-19-12-15(10-11-17(19)20)23-13-14-6-2-1-3-7-14/h1-12H,13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50385962

(CHEMBL2042461)Show SMILES COc1cc(Nc2cc3ccccc3cc2C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C25H21NO4/c1-29-24-15-20(11-12-23(24)30-16-17-7-3-2-4-8-17)26-22-14-19-10-6-5-9-18(19)13-21(22)25(27)28/h2-15,26H,16H2,1H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50385966

(CHEMBL2042465)Show InChI InChI=1S/C20H15NO2/c22-20-16-8-4-5-9-18(16)21-19-12-15(10-11-17(19)20)23-13-14-6-2-1-3-7-14/h1-12H,13H2,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291455

(N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC23CC(C2)(CC3)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |(-.82,-6.28,;-2.15,-5.51,;-3.48,-6.28,;-4.82,-5.51,;-4.82,-3.97,;-3.48,-3.2,;-3.48,-1.66,;-4.82,-.89,;-4.82,.65,;-6.28,1.13,;-5.88,-.36,;-7.19,-.12,;-6.28,-1.37,;-6.68,2.61,;-5.59,3.7,;-4.1,3.3,;-5.99,5.19,;-4.9,6.28,;-2.15,-3.97,;-.82,-3.2,;.52,-3.97,;1.85,-3.2,;1.85,-1.66,;3.19,-.89,;4.52,-1.66,;4.52,-3.2,;5.85,-3.97,;7.19,-3.2,;7.19,-1.66,;5.85,-.89,;.52,-.89,;-.82,-1.66,)| Show InChI InChI=1S/C25H25N5O2/c1-2-20(31)29-24-12-13-25(14-24,15-24)30-23-21(22(26)27-16-28-23)17-8-10-19(11-9-17)32-18-6-4-3-5-7-18/h2-11,16H,1,12-15H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611873
(CHEMBL5274020)Show SMILES CC(=C)\C=C\c1c(O)cc2oc3cc(O)c(O)c(\C=C\C(C)=C)c3c(=O)c2c1O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50385977

(CHEMBL2042446)Show InChI InChI=1S/C19H19NO/c1-2-3-6-14-9-11-15(12-10-14)18-13-19(21)16-7-4-5-8-17(16)20-18/h4-5,7-13H,2-3,6H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot anal... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291452
(N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...)Show SMILES Nc1ncnc(N[C@H]2CCC[C@H](C2)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-5,9-14,16,18-19H,1,6-8,15H2,(H,29,31)(H3,26,27,28,30)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50385962

(CHEMBL2042461)Show SMILES COc1cc(Nc2cc3ccccc3cc2C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C25H21NO4/c1-29-24-15-20(11-12-23(24)30-16-17-7-3-2-4-8-17)26-22-14-19-10-6-5-9-18(19)13-21(22)25(27)28/h2-15,26H,16H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50385984

(CHEMBL2042454)Show SMILES OC(=O)c1cc2ccccc2cc1Nc1ccc(OCc2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H18N2O5/c27-24(28)20-12-17-8-4-5-9-18(17)13-21(20)25-19-10-11-23(22(14-19)26(29)30)31-15-16-6-2-1-3-7-16/h1-14,25H,15H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy... |
Eur J Med Chem 54: 10-21 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.002
BindingDB Entry DOI: 10.7270/Q22J6CW7 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611878

(CHEMBL5273304)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc2oc3c(-[#8])ccc(-[#8])c3c(=O)c2c1-[#8] | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611869
(CHEMBL5268563)Show SMILES COc1c(O)cc2oc3cc(O)c(\C=C\C(C)=C)c(O)c3c(=O)c2c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611871
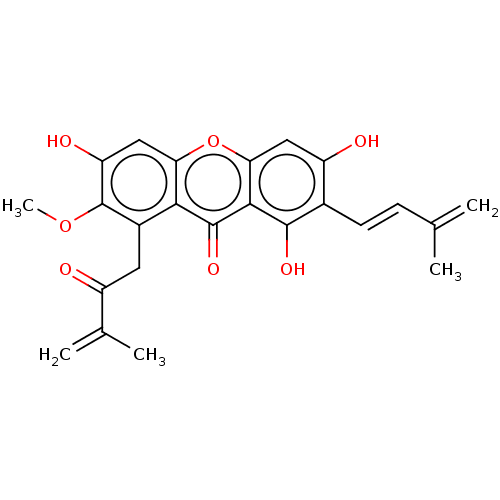
(CHEMBL5271556)Show SMILES COc1c(O)cc2oc3cc(O)c(\C=C\C(C)=C)c(O)c3c(=O)c2c1CC(=O)C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611877

(CHEMBL5287666)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(\[#6]=[#6]\[#6](-[#6])=[#6])c(-[#8])c2c1oc1c(-[#8])ccc(-[#8])c1c2=O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611876
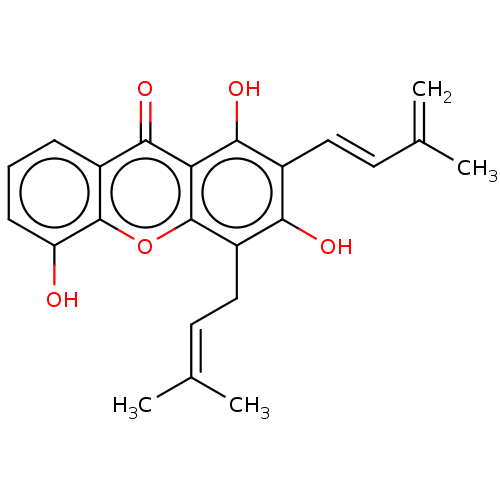
(CHEMBL5275750)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(\[#6]=[#6]\[#6](-[#6])=[#6])c(-[#8])c2c1oc1c(-[#8])cccc1c2=O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611870
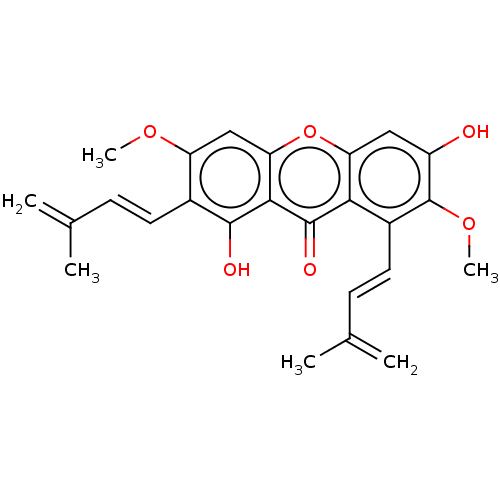
(CHEMBL5289397)Show SMILES COc1cc2oc3cc(O)c(OC)c(\C=C\C(C)=C)c3c(=O)c2c(O)c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438269
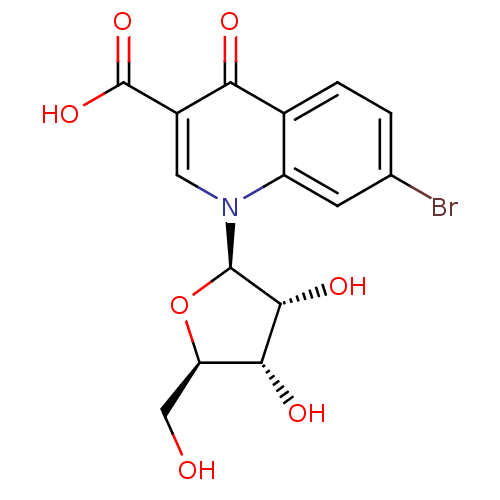
(CHEMBL2408381)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C(O)=O)c(=O)c2ccc(Br)cc12 |r| Show InChI InChI=1S/C15H14BrNO7/c16-6-1-2-7-9(3-6)17(4-8(11(7)19)15(22)23)14-13(21)12(20)10(5-18)24-14/h1-4,10,12-14,18,20-21H,5H2,(H,22,23)/t10-,12-,13-,14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Arginase
(Leishmania amazonensis) | BDBM50510706
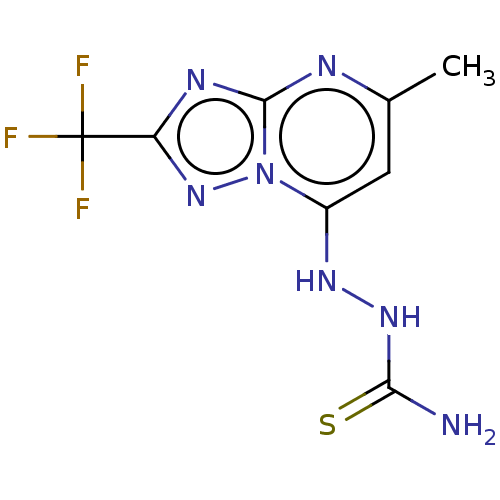
(CHEMBL4515068)Show InChI InChI=1S/C8H8F3N7S/c1-3-2-4(15-16-6(12)19)18-7(13-3)14-5(17-18)8(9,10)11/h2,15H,1H3,(H3,12,16,19) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 mins |
Bioorg Med Chem 27: 3061-3069 (2019)
Article DOI: 10.1016/j.bmc.2019.05.026
BindingDB Entry DOI: 10.7270/Q2SX6HJQ |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438274

(CHEMBL2408382)Show SMILES Cc1ccc2c(c1)n(cc(C(O)=O)c2=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO7/c1-7-2-3-8-10(4-7)17(5-9(12(8)19)16(22)23)15-14(21)13(20)11(6-18)24-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50325677
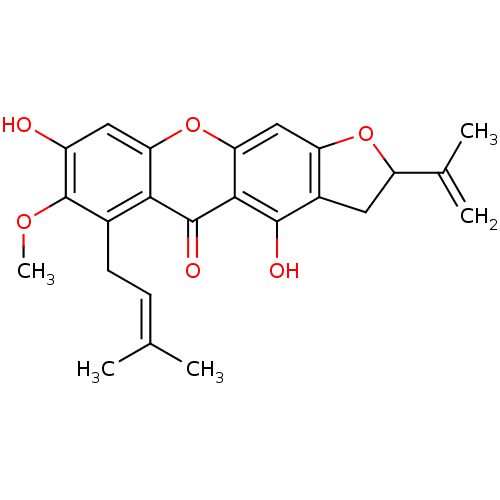
(4,8-dihydroxy-7-methoxy-6-(3-methylbut-2-enyl)-2-(...)Show SMILES [#6]-[#8]-c1c(-[#8])cc2oc3cc4-[#8]-[#6](-[#6]-c4c(-[#8])c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])=[#6] Show InChI InChI=1S/C24H24O6/c1-11(2)6-7-13-20-18(9-15(25)24(13)28-5)30-19-10-17-14(8-16(29-17)12(3)4)22(26)21(19)23(20)27/h6,9-10,16,25-26H,3,7-8H2,1-2,4-5H3 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438268
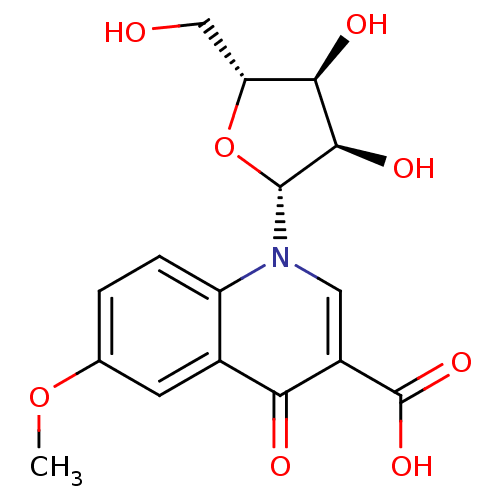
(CHEMBL2408383)Show SMILES COc1ccc2n(cc(C(O)=O)c(=O)c2c1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO8/c1-24-7-2-3-10-8(4-7)12(19)9(16(22)23)5-17(10)15-14(21)13(20)11(6-18)25-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611881
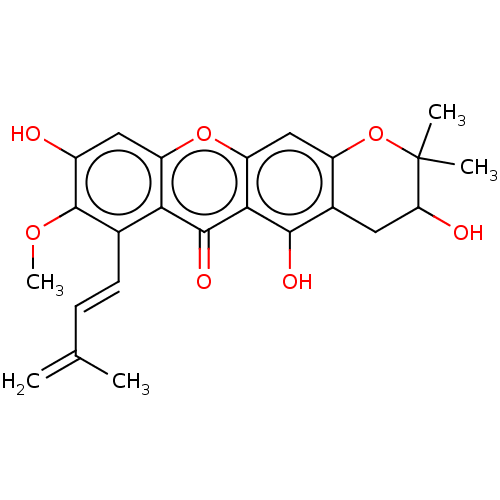
(CHEMBL5273562)Show SMILES COc1c(O)cc2oc3cc4OC(C)(C)C(O)Cc4c(O)c3c(=O)c2c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438273
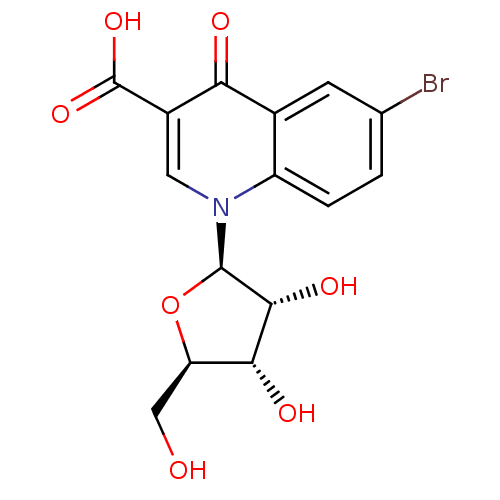
(CHEMBL2408384)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C(O)=O)c(=O)c2cc(Br)ccc12 |r| Show InChI InChI=1S/C15H14BrNO7/c16-6-1-2-9-7(3-6)11(19)8(15(22)23)4-17(9)14-13(21)12(20)10(5-18)24-14/h1-4,10,12-14,18,20-21H,5H2,(H,22,23)/t10-,12-,13-,14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438272

(CHEMBL560455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C(O)=O)c(=O)c2cc(Cl)ccc12 |r| Show InChI InChI=1S/C15H14ClNO7/c16-6-1-2-9-7(3-6)11(19)8(15(22)23)4-17(9)14-13(21)12(20)10(5-18)24-14/h1-4,10,12-14,18,20-21H,5H2,(H,22,23)/t10-,12-,13-,14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438271

(CHEMBL2408385)Show SMILES Cc1ccc2n(cc(C(O)=O)c(=O)c2c1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO7/c1-7-2-3-10-8(4-7)12(19)9(16(22)23)5-17(10)15-14(21)13(20)11(6-18)24-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438270
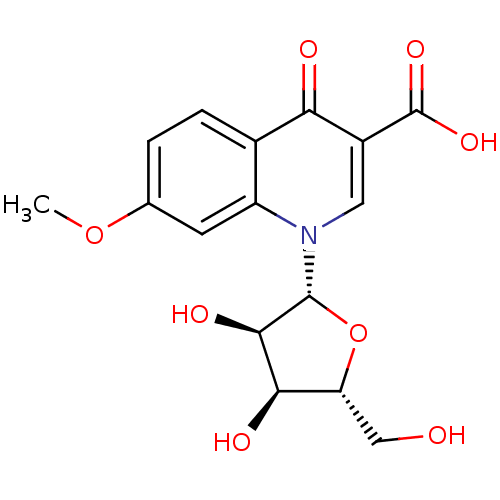
(CHEMBL2408386)Show SMILES COc1ccc2c(c1)n(cc(C(O)=O)c2=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO8/c1-24-7-2-3-8-10(4-7)17(5-9(12(8)19)16(22)23)15-14(21)13(20)11(6-18)25-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438270

(CHEMBL2408386)Show SMILES COc1ccc2c(c1)n(cc(C(O)=O)c2=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO8/c1-24-7-2-3-8-10(4-7)17(5-9(12(8)19)16(22)23)15-14(21)13(20)11(6-18)25-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438269

(CHEMBL2408381)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C(O)=O)c(=O)c2ccc(Br)cc12 |r| Show InChI InChI=1S/C15H14BrNO7/c16-6-1-2-7-9(3-6)17(4-8(11(7)19)15(22)23)14-13(21)12(20)10(5-18)24-14/h1-4,10,12-14,18,20-21H,5H2,(H,22,23)/t10-,12-,13-,14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase, glycosomal
(Trypanosoma cruzi) | BDBM50438268

(CHEMBL2408383)Show SMILES COc1ccc2n(cc(C(O)=O)c(=O)c2c1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17NO8/c1-24-7-2-3-10-8(4-7)12(19)9(16(22)23)5-17(10)15-14(21)13(20)11(6-18)25-15/h2-5,11,13-15,18,20-21H,6H2,1H3,(H,22,23)/t11-,13-,14-,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
da Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC method |
Bioorg Med Chem Lett 23: 4597-601 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.029
BindingDB Entry DOI: 10.7270/Q2Q52R15 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205961
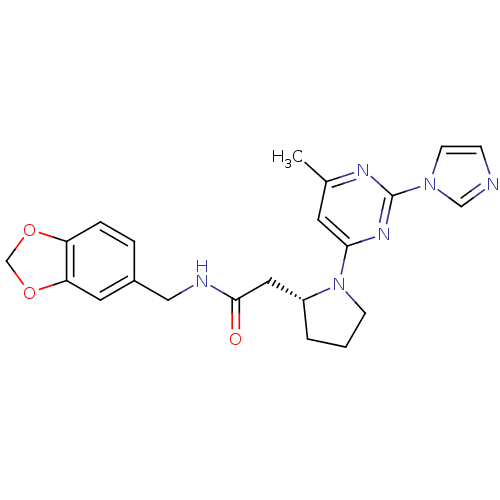
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES Cc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c1-15-9-20(26-22(25-15)27-8-6-23-13-27)28-7-2-3-17(28)11-21(29)24-12-16-4-5-18-19(10-16)31-14-30-18/h4-6,8-10,13,17H,2-3,7,11-12,14H2,1H3,(H,24,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205952
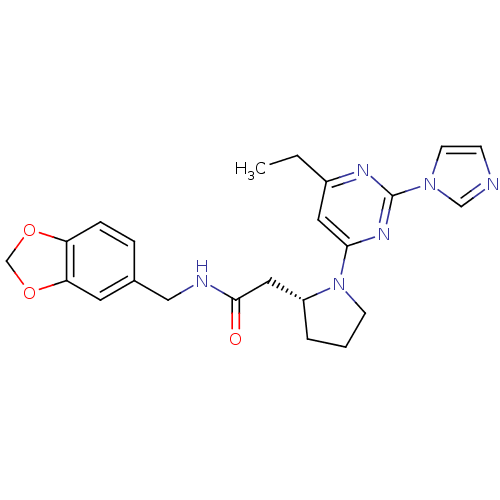
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES CCc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C23H26N6O3/c1-2-17-11-21(27-23(26-17)28-9-7-24-14-28)29-8-3-4-18(29)12-22(30)25-13-16-5-6-19-20(10-16)32-15-31-19/h5-7,9-11,14,18H,2-4,8,12-13,15H2,1H3,(H,25,30)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291635
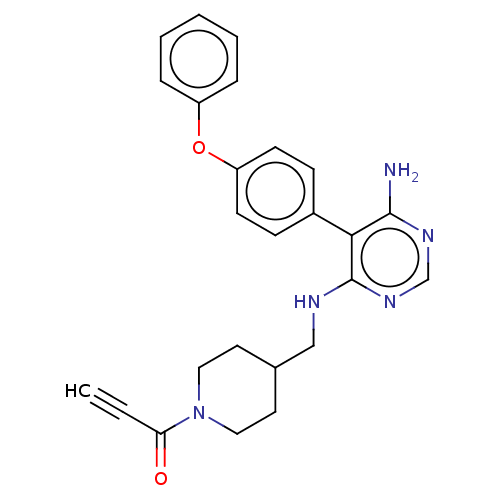
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C#C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h1,3-11,17-18H,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50519156
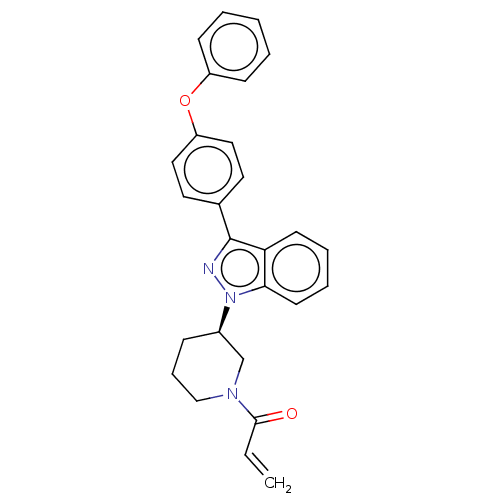
(CHEMBL4466205)Show SMILES C=CC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2)c2ccccc12 |r| Show InChI InChI=1S/C27H25N3O2/c1-2-26(31)29-18-8-9-21(19-29)30-25-13-7-6-12-24(25)27(28-30)20-14-16-23(17-15-20)32-22-10-4-3-5-11-22/h2-7,10-17,21H,1,8-9,18-19H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205910
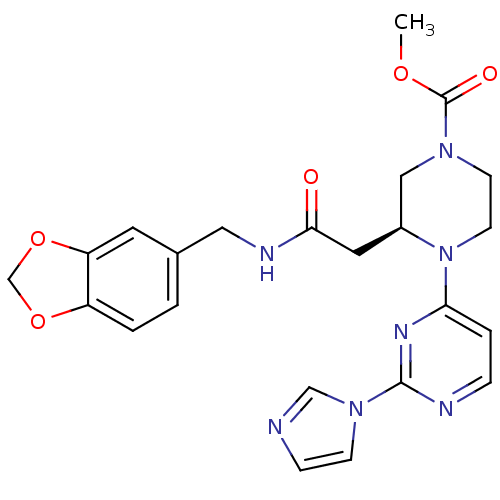
((3S)-methyl 4-(2-(1H-imidazol-1-yl)pyrimidin-4-yl)...)Show SMILES COC(=O)N1CCN([C@@H](CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205937
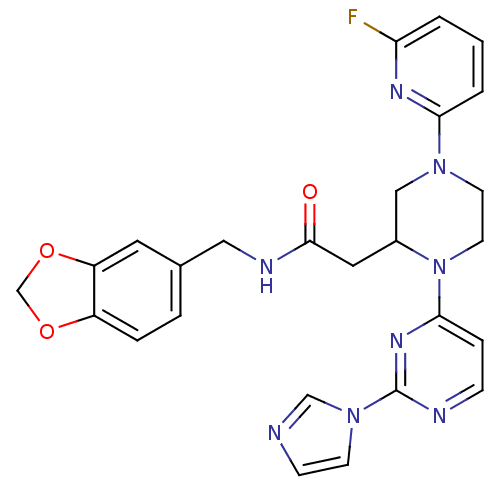
(CHEMBL385325 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES Fc1cccc(n1)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C26H25FN8O3/c27-22-2-1-3-23(31-22)33-10-11-35(24-6-7-29-26(32-24)34-9-8-28-16-34)19(15-33)13-25(36)30-14-18-4-5-20-21(12-18)38-17-37-20/h1-9,12,16,19H,10-11,13-15,17H2,(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205950
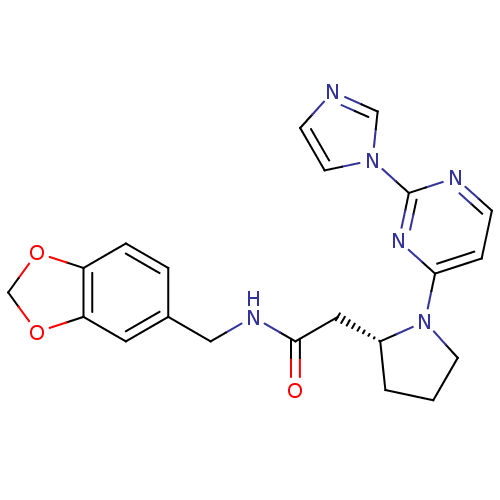
(2-((R)-1-(2-(1H-imidazol-1-yl)pyrimidin-4-yl)pyrro...)Show SMILES O=C(C[C@H]1CCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C21H22N6O3/c28-20(24-12-15-3-4-17-18(10-15)30-14-29-17)11-16-2-1-8-27(16)19-5-6-23-21(25-19)26-9-7-22-13-26/h3-7,9-10,13,16H,1-2,8,11-12,14H2,(H,24,28)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205947

((2R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4-y...)Show SMILES Cc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1C(=O)NCCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c1-15-11-20(26-22(25-15)27-10-8-23-13-27)28-9-2-3-17(28)21(29)24-7-6-16-4-5-18-19(12-16)31-14-30-18/h4-5,8,10-13,17H,2-3,6-7,9,14H2,1H3,(H,24,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50111438
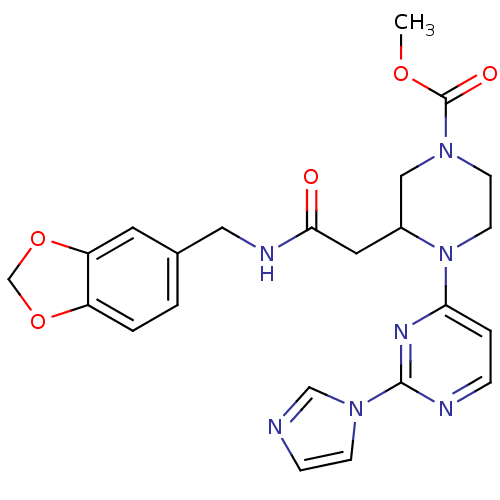
(3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...)Show SMILES COC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205932

(CHEMBL373623 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CN1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C22H25N7O3/c1-27-8-9-29(20-4-5-24-22(26-20)28-7-6-23-14-28)17(13-27)11-21(30)25-12-16-2-3-18-19(10-16)32-15-31-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205911
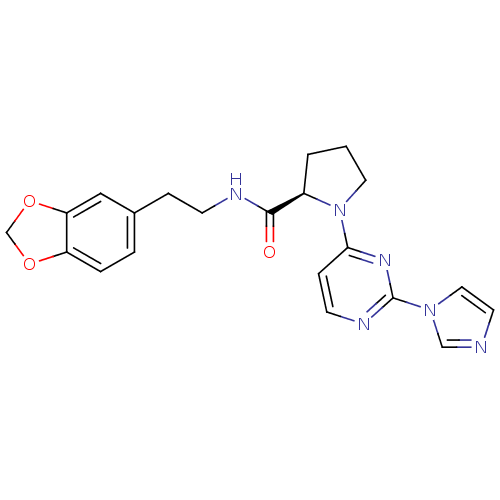
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES O=C(NCCc1ccc2OCOc2c1)[C@H]1CCCN1c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C21H22N6O3/c28-20(23-7-5-15-3-4-17-18(12-15)30-14-29-17)16-2-1-10-27(16)19-6-8-24-21(25-19)26-11-9-22-13-26/h3-4,6,8-9,11-13,16H,1-2,5,7,10,14H2,(H,23,28)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205956
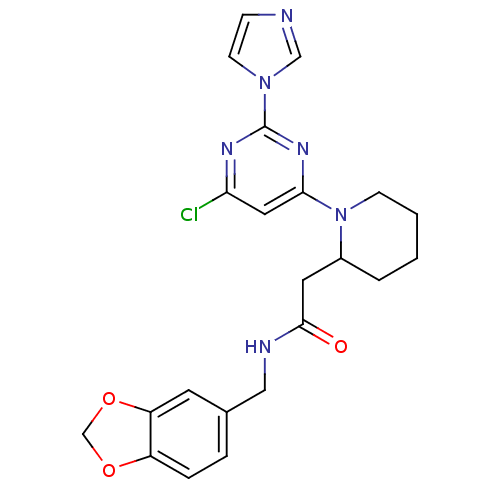
(CHEMBL223788 | N-(1,3-benzodioxol-5-ylmethyl)-1-[6...)Show SMILES Clc1cc(nc(n1)-n1ccnc1)N1CCCCC1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H23ClN6O3/c23-19-11-20(27-22(26-19)28-8-6-24-13-28)29-7-2-1-3-16(29)10-21(30)25-12-15-4-5-17-18(9-15)32-14-31-17/h4-6,8-9,11,13,16H,1-3,7,10,12,14H2,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205954

(CHEMBL385334 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)c1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C28H27N7O4/c36-26(31-16-20-6-7-23-24(14-20)39-19-38-23)15-22-17-33(27(37)21-4-2-1-3-5-21)12-13-35(22)25-8-9-30-28(32-25)34-11-10-29-18-34/h1-11,14,18,22H,12-13,15-17,19H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205926
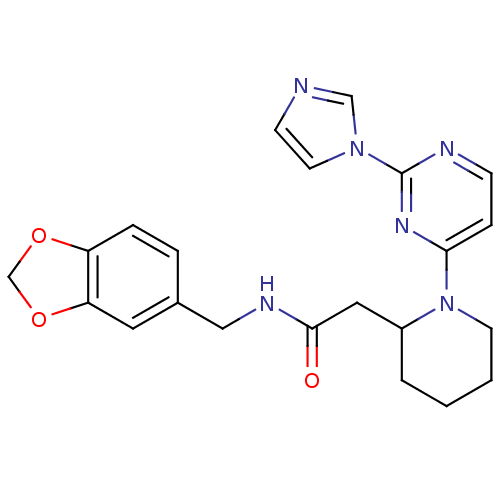
(CHEMBL442041 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CCCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c29-21(25-13-16-4-5-18-19(11-16)31-15-30-18)12-17-3-1-2-9-28(17)20-6-7-24-22(26-20)27-10-8-23-14-27/h4-8,10-11,14,17H,1-3,9,12-13,15H2,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data