 Found 9318 hits with Last Name = 'yu' and Initial = 'c'
Found 9318 hits with Last Name = 'yu' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479434
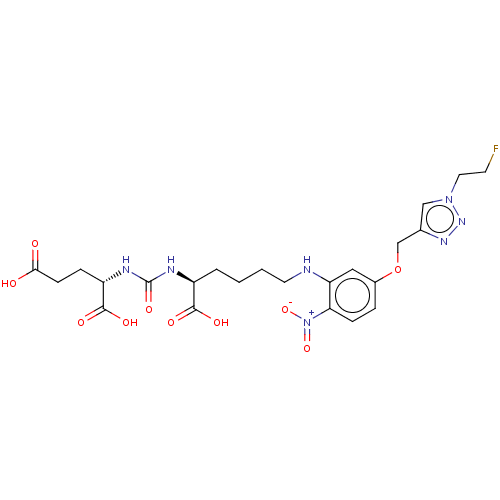
(US10894807, ID P242)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNc1cc(OCc2cn(CCF)nn2)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H30FN7O10/c24-8-10-30-12-14(28-29-30)13-41-15-4-6-19(31(39)40)18(11-15)25-9-2-1-3-16(21(34)35)26-23(38)27-17(22(36)37)5-7-20(32)33/h4,6,11-12,16-17,25H,1-3,5,7-10,13H2,(H,32,33)(H,34,35)(H,36,37)(H2,26,27,38)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass... |
ACS Med Chem Lett 7: 100-4 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00428
BindingDB Entry DOI: 10.7270/Q25T3NCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479437
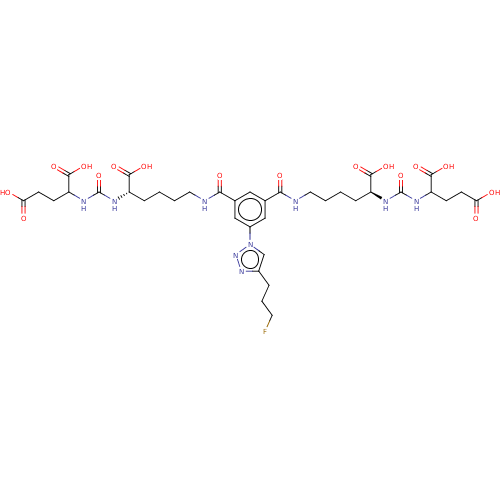
(US10894807, ID P246)Show SMILES OC(=O)CCC(NC(=O)N[C@@H](CCCCNC(=O)c1cc(cc(c1)-n1cc(CCCF)nn1)C(=O)NCCCC[C@H](NC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H50FN9O16/c38-13-5-6-22-19-47(46-45-22)23-17-20(30(52)39-14-3-1-7-24(32(54)55)41-36(62)43-26(34(58)59)9-11-28(48)49)16-21(18-23)31(53)40-15-4-2-8-25(33(56)57)42-37(63)44-27(35(60)61)10-12-29(50)51/h16-19,24-27H,1-15H2,(H,39,52)(H,40,53)(H,48,49)(H,50,51)(H,54,55)(H,56,57)(H,58,59)(H,60,61)(H2,41,43,62)(H2,42,44,63)/t24-,25-,26?,27?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479450
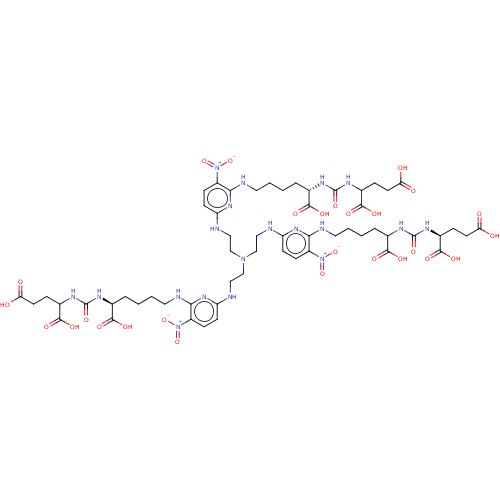
(US10894807, ID P270)Show SMILES OC(=O)CCC(NC(=O)N[C@@H](CCCCNc1nc(NCCN(CCNc2ccc(c(NCCCCC(NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)n2)[N+]([O-])=O)CCNc2ccc(c(NCCCC[C@H](NC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)n2)[N+]([O-])=O)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C57H81N19O27/c77-43(78)19-10-34(52(89)90)67-55(95)64-31(49(83)84)7-1-4-22-61-46-37(74(98)99)13-16-40(70-46)58-25-28-73(29-26-59-41-17-14-38(75(100)101)47(71-41)62-23-5-2-8-32(50(85)86)65-56(96)68-35(53(91)92)11-20-44(79)80)30-27-60-42-18-15-39(76(102)103)48(72-42)63-24-6-3-9-33(51(87)88)66-57(97)69-36(54(93)94)12-21-45(81)82/h13-18,31-36H,1-12,19-30H2,(H,77,78)(H,79,80)(H,81,82)(H,83,84)(H,85,86)(H,87,88)(H,89,90)(H,91,92)(H,93,94)(H2,58,61,70)(H2,59,62,71)(H2,60,63,72)(H2,64,67,95)(H2,65,68,96)(H2,66,69,97)/t31-,32-,33?,34?,35?,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479453
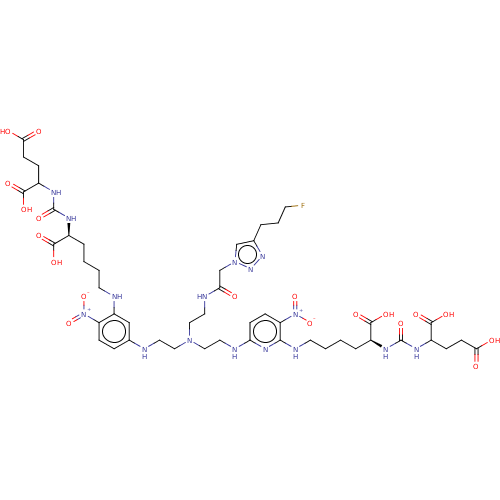
(US10894807, ID P273)Show SMILES OC(=O)CCC(NC(=O)N[C@@H](CCCCNc1cc(NCCN(CCNC(=O)Cn2cc(CCCF)nn2)CCNc2ccc(c(NCCCC[C@H](NC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)n2)[N+]([O-])=O)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C48H69FN16O19/c49-17-5-6-30-27-63(61-60-30)28-39(66)53-22-25-62(23-20-50-29-9-12-36(64(81)82)35(26-29)51-18-3-1-7-31(43(71)72)55-47(79)57-33(45(75)76)10-15-40(67)68)24-21-52-38-14-13-37(65(83)84)42(59-38)54-19-4-2-8-32(44(73)74)56-48(80)58-34(46(77)78)11-16-41(69)70/h9,12-14,26-27,31-34,50-51H,1-8,10-11,15-25,28H2,(H,53,66)(H,67,68)(H,69,70)(H,71,72)(H,73,74)(H,75,76)(H,77,78)(H2,52,54,59)(H2,55,57,79)(H2,56,58,80)/t31-,32-,33?,34?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479444
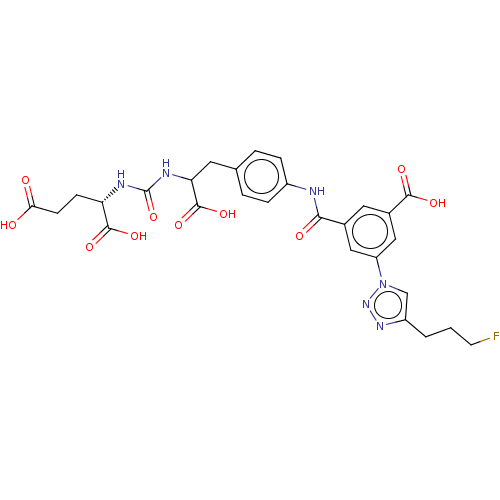
(US10894807, ID P253)Show SMILES OC(=O)CC[C@H](NC(=O)NC(Cc1ccc(NC(=O)c2cc(cc(c2)-n2cc(CCCF)nn2)C(O)=O)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H29FN6O10/c29-9-1-2-19-14-35(34-33-19)20-12-16(11-17(13-20)25(39)40)24(38)30-18-5-3-15(4-6-18)10-22(27(43)44)32-28(45)31-21(26(41)42)7-8-23(36)37/h3-6,11-14,21-22H,1-2,7-10H2,(H,30,38)(H,36,37)(H,39,40)(H,41,42)(H,43,44)(H2,31,32,45)/t21-,22?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636
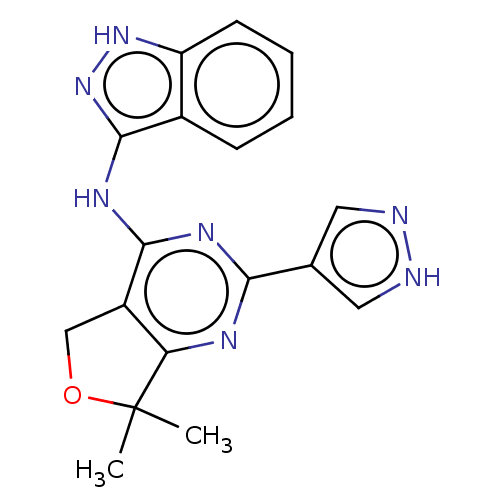
(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479458
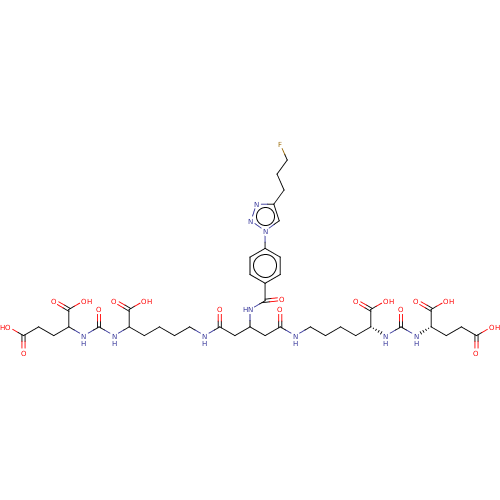
(US10894807, ID P278)Show SMILES OC(=O)CCC(NC(=O)NC(CCCCNC(=O)CC(CC(=O)NCCCC[C@@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)NC(=O)c1ccc(cc1)-n1cc(CCCF)nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C41H57FN10O17/c42-17-5-6-24-22-52(51-50-24)26-11-9-23(10-12-26)35(59)45-25(20-31(53)43-18-3-1-7-27(36(60)61)46-40(68)48-29(38(64)65)13-15-33(55)56)21-32(54)44-19-4-2-8-28(37(62)63)47-41(69)49-30(39(66)67)14-16-34(57)58/h9-12,22,25,27-30H,1-8,13-21H2,(H,43,53)(H,44,54)(H,45,59)(H,55,56)(H,57,58)(H,60,61)(H,62,63)(H,64,65)(H,66,67)(H2,46,48,68)(H2,47,49,69)/t25?,27-,28?,29+,30?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408675
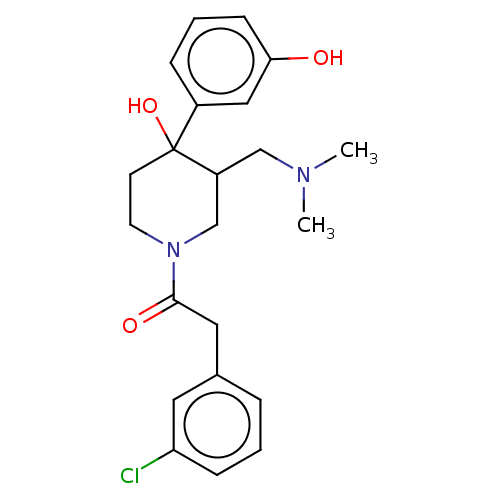
(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479431

(US10894807, ID P238)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](Cc1ccc(NC(=O)c2cc(cc(c2)-n2cc(CCCF)nn2)C(=O)Nc2ccc(C[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)cc2)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C43H46FN9O16/c44-15-1-2-28-21-53(52-51-28)29-19-24(36(58)45-26-7-3-22(4-8-26)16-32(40(64)65)49-42(68)47-30(38(60)61)11-13-34(54)55)18-25(20-29)37(59)46-27-9-5-23(6-10-27)17-33(41(66)67)50-43(69)48-31(39(62)63)12-14-35(56)57/h3-10,18-21,30-33H,1-2,11-17H2,(H,45,58)(H,46,59)(H,54,55)(H,56,57)(H,60,61)(H,62,63)(H,64,65)(H,66,67)(H2,47,49,68)(H2,48,50,69)/t30-,31-,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479415

(US10894807, ID P200)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=S)Nc1ccc(cc1)-n1cc(CCCF)nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H32FN7O7S/c25-12-3-4-16-14-32(31-30-16)17-8-6-15(7-9-17)27-24(40)26-13-2-1-5-18(21(35)36)28-23(39)29-19(22(37)38)10-11-20(33)34/h6-9,14,18-19H,1-5,10-13H2,(H,33,34)(H,35,36)(H,37,38)(H2,26,27,40)(H2,28,29,39)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
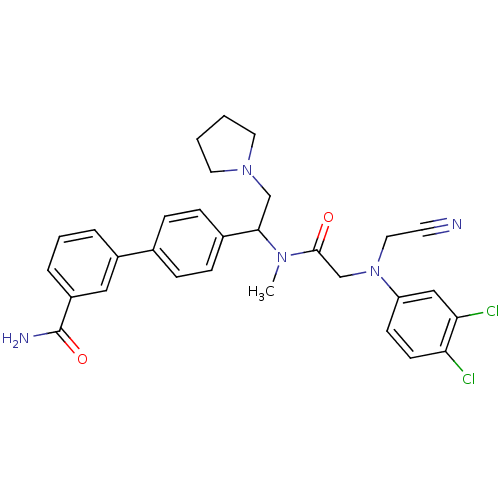
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479429
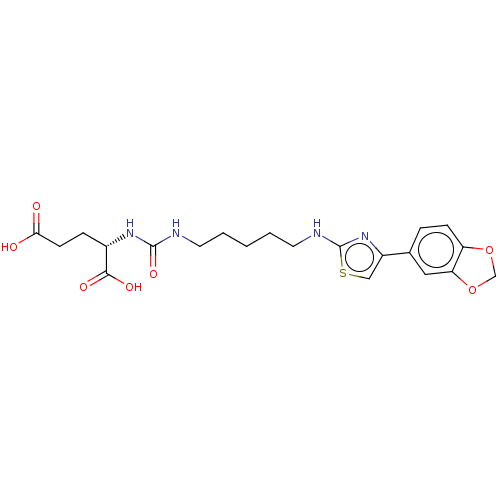
(US10894807, ID P235)Show SMILES OC(=O)CC[C@H](NC(=O)NCCCCCNc1nc(cs1)-c1ccc2OCOc2c1)C(O)=O |r| Show InChI InChI=1S/C21H26N4O7S/c26-18(27)7-5-14(19(28)29)24-20(30)22-8-2-1-3-9-23-21-25-15(11-33-21)13-4-6-16-17(10-13)32-12-31-16/h4,6,10-11,14H,1-3,5,7-9,12H2,(H,23,25)(H,26,27)(H,28,29)(H2,22,24,30)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479449

(US10894807, ID P266)Show SMILES OC(=O)CC[C@@H](NC(=O)N[C@H](CCCCNc1cc(OCC(COc2ccc(c(NCCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c2)[N+]([O-])=O)n2cc(CCCF)nn2)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C44H58FN11O20/c45-17-5-6-25-22-54(53-52-25)26(23-75-27-9-13-35(55(71)72)33(20-27)46-18-3-1-7-29(39(61)62)48-43(69)50-31(41(65)66)11-15-37(57)58)24-76-28-10-14-36(56(73)74)34(21-28)47-19-4-2-8-30(40(63)64)49-44(70)51-32(42(67)68)12-16-38(59)60/h9-10,13-14,20-22,26,29-32,46-47H,1-8,11-12,15-19,23-24H2,(H,57,58)(H,59,60)(H,61,62)(H,63,64)(H,65,66)(H,67,68)(H2,48,50,69)(H2,49,51,70)/t26?,29-,30+,31-,32+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479442
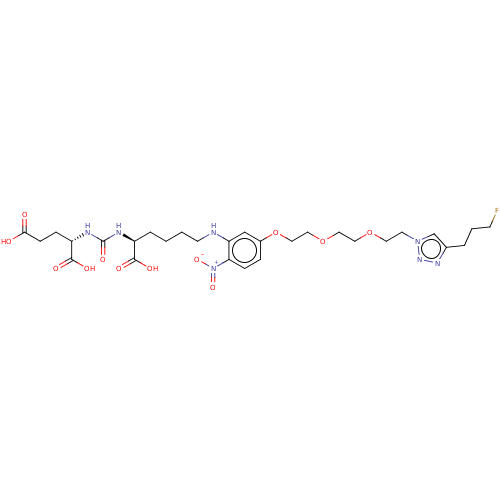
(US10894807, ID P251)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNc1cc(OCCOCCOCCn2cc(CCCF)nn2)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H42FN7O12/c30-10-3-4-20-19-36(35-34-20)12-13-47-14-15-48-16-17-49-21-6-8-25(37(45)46)24(18-21)31-11-2-1-5-22(27(40)41)32-29(44)33-23(28(42)43)7-9-26(38)39/h6,8,18-19,22-23,31H,1-5,7,9-17H2,(H,38,39)(H,40,41)(H,42,43)(H2,32,33,44)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695
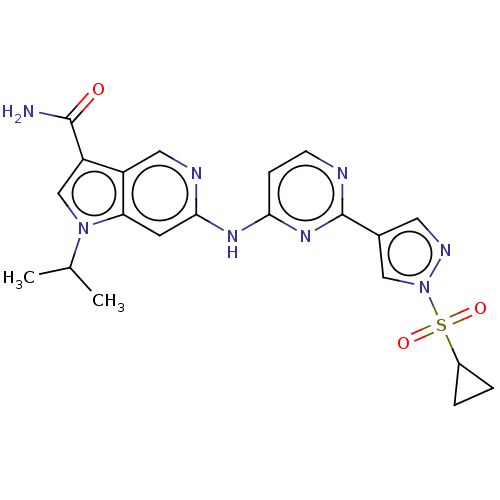
(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479433
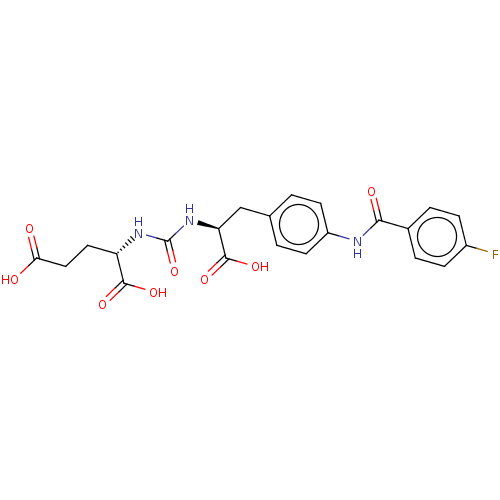
(US10894807, ID P241)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](Cc1ccc(NC(=O)c2ccc(F)cc2)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H22FN3O8/c23-14-5-3-13(4-6-14)19(29)24-15-7-1-12(2-8-15)11-17(21(32)33)26-22(34)25-16(20(30)31)9-10-18(27)28/h1-8,16-17H,9-11H2,(H,24,29)(H,27,28)(H,30,31)(H,32,33)(H2,25,26,34)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479435
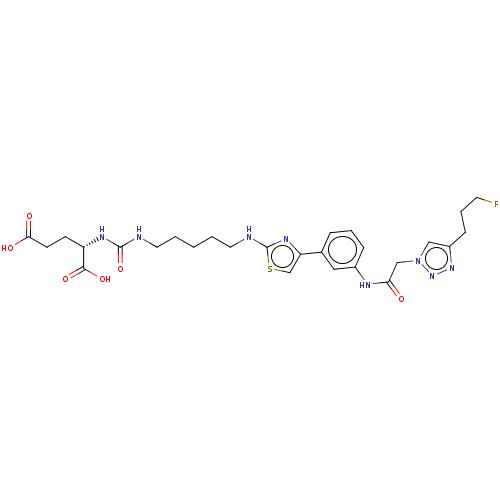
(US10894807, ID P244)Show SMILES OC(=O)CC[C@H](NC(=O)NCCCCCNc1nc(cs1)-c1cccc(NC(=O)Cn2cc(CCCF)nn2)c1)C(O)=O |r| Show InChI InChI=1S/C27H35FN8O6S/c28-11-5-8-20-15-36(35-34-20)16-23(37)31-19-7-4-6-18(14-19)22-17-43-27(33-22)30-13-3-1-2-12-29-26(42)32-21(25(40)41)9-10-24(38)39/h4,6-7,14-15,17,21H,1-3,5,8-13,16H2,(H,30,33)(H,31,37)(H,38,39)(H,40,41)(H2,29,32,42)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479457
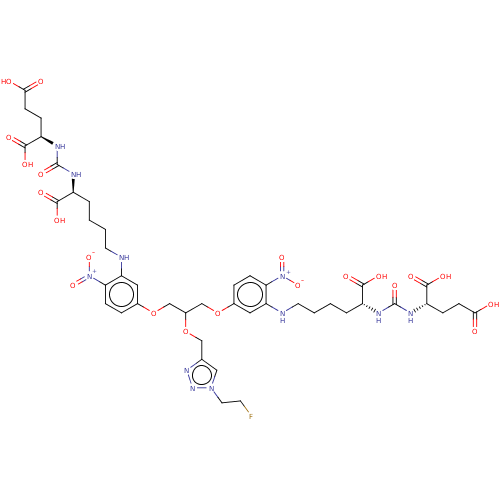
(US10894807, ID P277)Show SMILES OC(=O)CC[C@@H](NC(=O)N[C@@H](CCCCNc1cc(OCC(COc2ccc(c(NCCCC[C@@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c2)[N+]([O-])=O)OCc2cn(CCF)nn2)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C44H58FN11O21/c45-15-18-54-21-25(52-53-54)22-75-28(23-76-26-7-11-35(55(71)72)33(19-26)46-16-3-1-5-29(39(61)62)48-43(69)50-31(41(65)66)9-13-37(57)58)24-77-27-8-12-36(56(73)74)34(20-27)47-17-4-2-6-30(40(63)64)49-44(70)51-32(42(67)68)10-14-38(59)60/h7-8,11-12,19-21,28-32,46-47H,1-6,9-10,13-18,22-24H2,(H,57,58)(H,59,60)(H,61,62)(H,63,64)(H,65,66)(H,67,68)(H2,48,50,69)(H2,49,51,70)/t28?,29-,30+,31+,32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479445
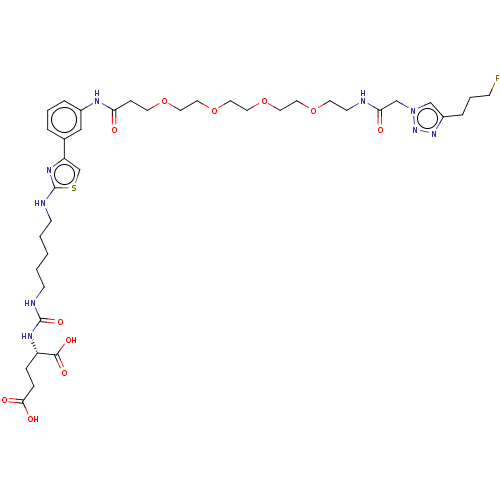
(US10894807, ID P254)Show SMILES OC(=O)CC[C@H](NC(=O)NCCCCCNc1nc(cs1)-c1cccc(NC(=O)CCOCCOCCOCCOCCNC(=O)Cn2cc(CCCF)nn2)c1)C(O)=O |r| Show InChI InChI=1S/C38H56FN9O11S/c39-12-5-8-30-25-48(47-46-30)26-34(50)40-15-17-57-19-21-59-23-22-58-20-18-56-16-11-33(49)43-29-7-4-6-28(24-29)32-27-60-38(45-32)42-14-3-1-2-13-41-37(55)44-31(36(53)54)9-10-35(51)52/h4,6-7,24-25,27,31H,1-3,5,8-23,26H2,(H,40,50)(H,42,45)(H,43,49)(H,51,52)(H,53,54)(H2,41,44,55)/t31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50209744
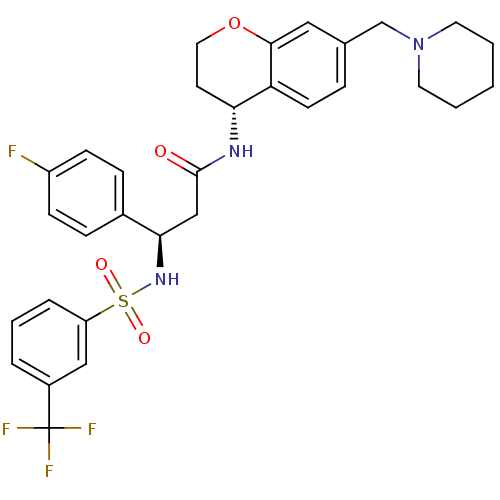
((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...)Show SMILES Fc1ccc(cc1)[C@@H](CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12)NS(=O)(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H33F4N3O4S/c32-24-10-8-22(9-11-24)28(37-43(40,41)25-6-4-5-23(18-25)31(33,34)35)19-30(39)36-27-13-16-42-29-17-21(7-12-26(27)29)20-38-14-2-1-3-15-38/h4-12,17-18,27-28,37H,1-3,13-16,19-20H2,(H,36,39)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells |
J Med Chem 50: 2200-12 (2007)
Article DOI: 10.1021/jm070055c
BindingDB Entry DOI: 10.7270/Q2MS3SG2 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377220
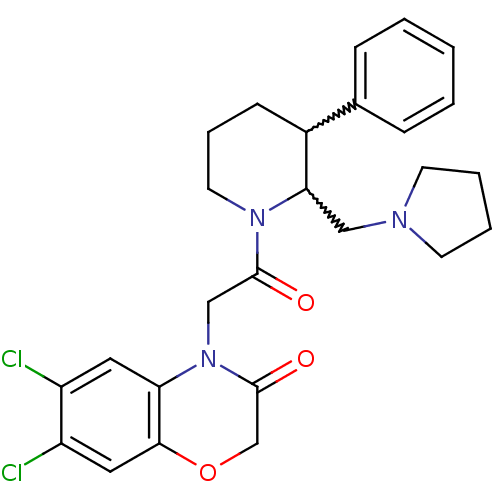
(CHEMBL255509)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H29Cl2N3O3/c27-20-13-22-24(14-21(20)28)34-17-26(33)31(22)16-25(32)30-12-6-9-19(18-7-2-1-3-8-18)23(30)15-29-10-4-5-11-29/h1-3,7-8,13-14,19,23H,4-6,9-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377218
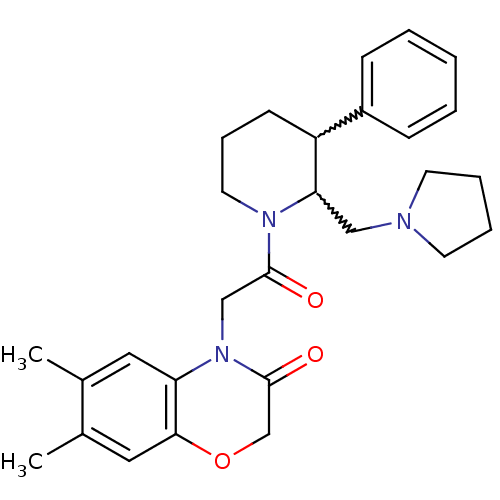
(CHEMBL257171)Show SMILES Cc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H35N3O3/c1-20-15-24-26(16-21(20)2)34-19-28(33)31(24)18-27(32)30-14-8-11-23(22-9-4-3-5-10-22)25(30)17-29-12-6-7-13-29/h3-5,9-10,15-16,23,25H,6-8,11-14,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463294

(CHEMBL4249256)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@H](C(=O)c4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,wU:16.16,1.0,wD:17.38,28.36,7.7,19.23,c:37,THB:10:9:17:5.6.4,(9.78,-11.07,;9.03,-9.74,;7.65,-10.81,;5.94,-9.74,;6.72,-8.4,;5.95,-7.07,;6.71,-5.74,;8.25,-5.74,;9.79,-5.74,;9.03,-4.41,;9.78,-3.07,;11.32,-3.05,;12.66,-3.81,;12.64,-2.27,;7.47,-5.14,;7.41,-7.18,;8.25,-8.41,;9.02,-7.07,;10.56,-7.06,;11.34,-8.4,;12.88,-8.4,;13.65,-7.07,;13.65,-9.73,;12.87,-11.06,;13.64,-12.4,;15.18,-12.4,;15.95,-11.05,;15.18,-9.72,;10.59,-9.7,;11.34,-11.07,;10.57,-12.41,;9.25,-8.93,;10.35,-7.84,;4.41,-7.06,;3.64,-8.38,;4.4,-9.72,;3.62,-11.05,;2.08,-11.04,)| Show InChI InChI=1S/C31H33NO4/c1-34-23-11-10-21-16-24-29-12-13-31(35-2,22(17-29)26(33)20-6-4-3-5-7-20)28-30(29,25(21)27(23)36-28)14-15-32(24)18-19-8-9-19/h3-7,10-13,19,22,24,28H,8-9,14-18H2,1-2H3/t22-,24-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141902

(CHEMBL3758602)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2c(Cl)cccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H16ClN7O/c1-18(2)14-11(8-27-18)16(23-15(22-14)9-6-20-21-7-9)24-17-10-4-3-5-12(19)13(10)25-26-17/h3-7H,8H2,1-2H3,(H,20,21)(H2,22,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
| Assay Description
The transfection of plasmid and membrane preparations was conducted as described in our previous report, with 0.7 nm [3H] SCH23390 (D1R) or [3H]Spipe... |
Chem Biol Drug Des 88: 599-607 (2016)
Article DOI: 10.1111/cbdd.12796
BindingDB Entry DOI: 10.7270/Q2QF8RQ5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610
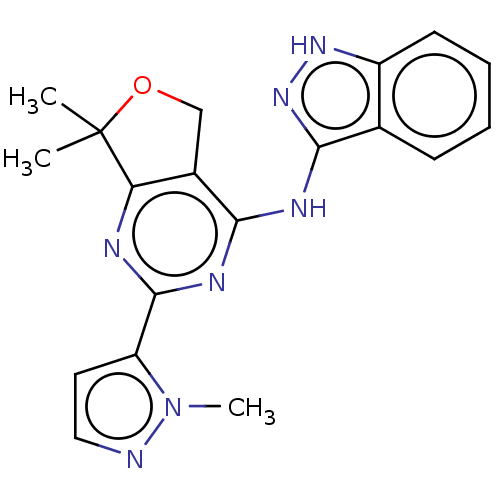
(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479464
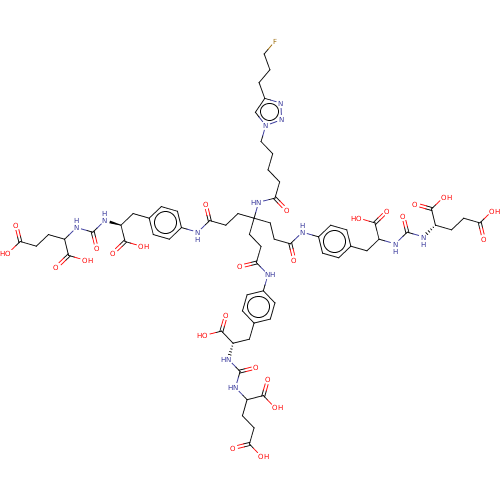
(US10894807, ID P285)Show SMILES OC(=O)CCC(NC(=O)N[C@@H](Cc1ccc(NC(=O)CCC(CCC(=O)Nc2ccc(CC(NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)cc2)(CCC(=O)Nc2ccc(C[C@H](NC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)cc2)NC(=O)CCCCn2cc(CCCF)nn2)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C65H82FN13O25/c66-30-3-4-42-35-79(78-77-42)31-2-1-5-52(83)76-65(27-24-49(80)67-39-12-6-36(7-13-39)32-46(59(96)97)73-62(102)70-43(56(90)91)18-21-53(84)85,28-25-50(81)68-40-14-8-37(9-15-40)33-47(60(98)99)74-63(103)71-44(57(92)93)19-22-54(86)87)29-26-51(82)69-41-16-10-38(11-17-41)34-48(61(100)101)75-64(104)72-45(58(94)95)20-23-55(88)89/h6-17,35,43-48H,1-5,18-34H2,(H,67,80)(H,68,81)(H,69,82)(H,76,83)(H,84,85)(H,86,87)(H,88,89)(H,90,91)(H,92,93)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H2,70,73,102)(H2,71,74,103)(H2,72,75,104)/t43-,44?,45?,46?,47-,48-,65?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50514929
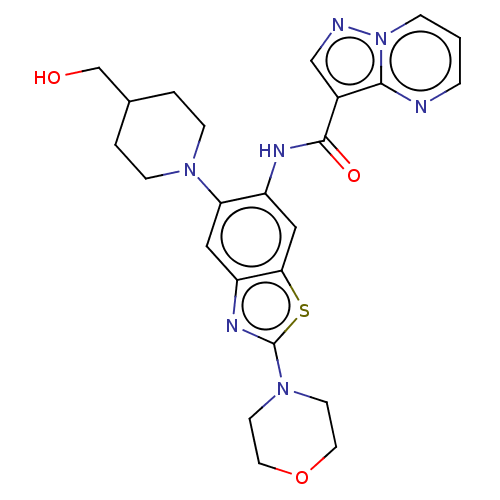
(CHEMBL4474636)Show SMILES OCC1CCN(CC1)c1cc2nc(sc2cc1NC(=O)c1cnn2cccnc12)N1CCOCC1 Show InChI InChI=1S/C24H27N7O3S/c32-15-16-2-6-29(7-3-16)20-12-19-21(35-24(28-19)30-8-10-34-11-9-30)13-18(20)27-23(33)17-14-26-31-5-1-4-25-22(17)31/h1,4-5,12-14,16,32H,2-3,6-11,15H2,(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... |
J Med Chem 62: 6223-6240 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00439
BindingDB Entry DOI: 10.7270/Q2CC1419 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50514930

(CHEMBL4464832)Show SMILES CN(C)c1nc2cc(N3CCC(CO)CC3)c(NC(=O)c3cnn4cccnc34)cc2s1 Show InChI InChI=1S/C22H25N7O2S/c1-27(2)22-26-17-10-18(28-8-4-14(13-30)5-9-28)16(11-19(17)32-22)25-21(31)15-12-24-29-7-3-6-23-20(15)29/h3,6-7,10-12,14,30H,4-5,8-9,13H2,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... |
J Med Chem 62: 6223-6240 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00439
BindingDB Entry DOI: 10.7270/Q2CC1419 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610

(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50498973
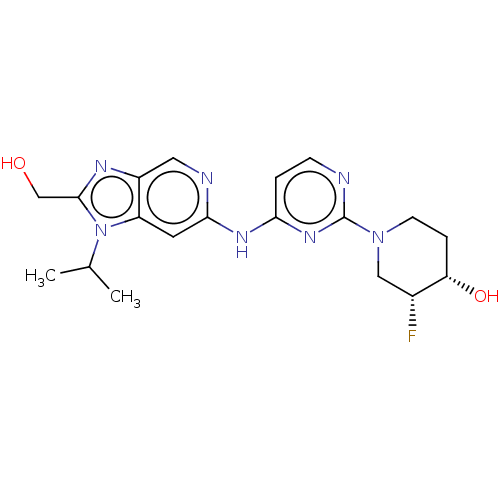
(CHEMBL3734934)Show SMILES CC(C)n1c(CO)nc2cnc(Nc3ccnc(n3)N3CC[C@H](O)[C@H](F)C3)cc12 |r| Show InChI InChI=1S/C19H24FN7O2/c1-11(2)27-14-7-17(22-8-13(14)23-18(27)10-28)24-16-3-5-21-19(25-16)26-6-4-15(29)12(20)9-26/h3,5,7-8,11-12,15,28-29H,4,6,9-10H2,1-2H3,(H,21,22,24,25)/t12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type EGFR using Fl-EEPLYWSFPAKKK-CONH2 as substrate preincubated for 30 mins followed by addition of substrate measured afte... |
J Med Chem 58: 8877-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01412
BindingDB Entry DOI: 10.7270/Q2833W1G |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479426
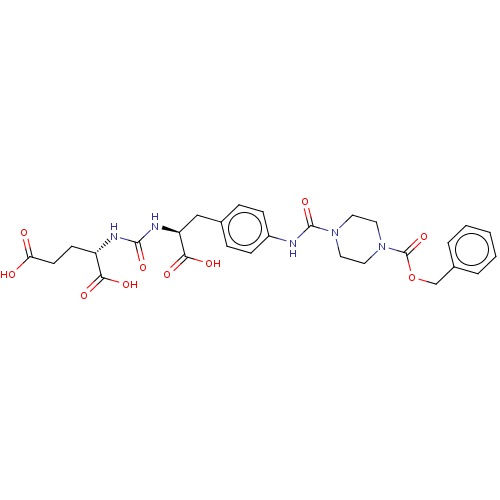
(US10894807, ID P222)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](Cc1ccc(NC(=O)N2CCN(CC2)C(=O)OCc2ccccc2)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H33N5O10/c34-23(35)11-10-21(24(36)37)30-26(40)31-22(25(38)39)16-18-6-8-20(9-7-18)29-27(41)32-12-14-33(15-13-32)28(42)43-17-19-4-2-1-3-5-19/h1-9,21-22H,10-17H2,(H,29,41)(H,34,35)(H,36,37)(H,38,39)(H2,30,31,40)/t21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479425
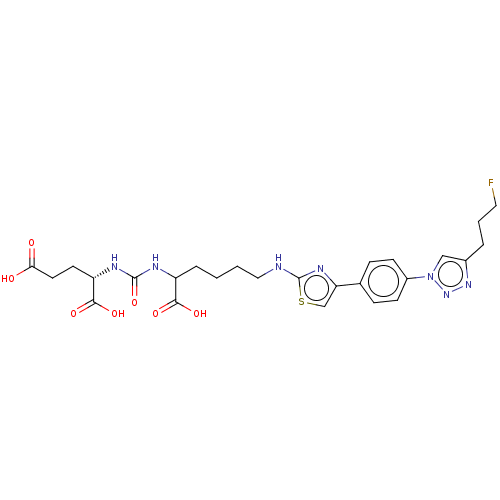
(US10894807, ID P218)Show SMILES OC(=O)CC[C@H](NC(=O)NC(CCCCNc1nc(cs1)-c1ccc(cc1)-n1cc(CCCF)nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H32FN7O7S/c27-12-3-4-17-14-34(33-32-17)18-8-6-16(7-9-18)21-15-42-26(31-21)28-13-2-1-5-19(23(37)38)29-25(41)30-20(24(39)40)10-11-22(35)36/h6-9,14-15,19-20H,1-5,10-13H2,(H,28,31)(H,35,36)(H,37,38)(H,39,40)(H2,29,30,41)/t19?,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377217
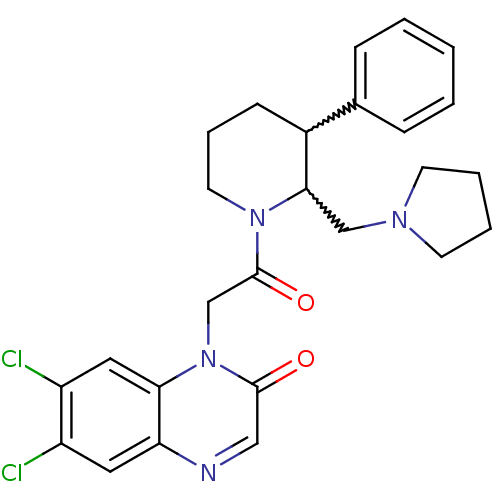
(CHEMBL256989)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H28Cl2N4O2/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-12-6-9-19(18-7-2-1-3-8-18)24(31)16-30-10-4-5-11-30/h1-3,7-8,13-15,19,24H,4-6,9-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613688

(CHEMBL5282716)Show SMILES CC(C)n1nc(N2CC(C2)C(C)(C)O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613687
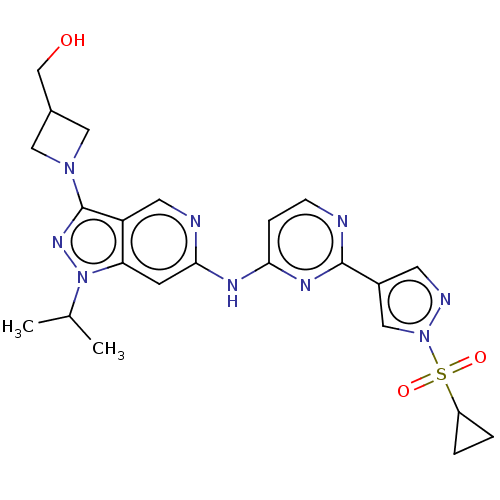
(CHEMBL5285503)Show SMILES CC(C)n1nc(N2CC(CO)C2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data