

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50493081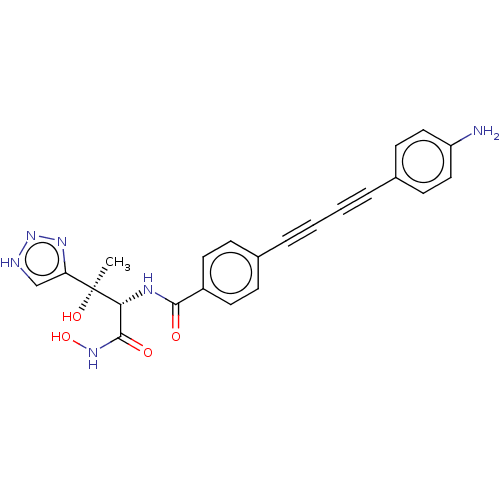 (CHEMBL2420205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50493080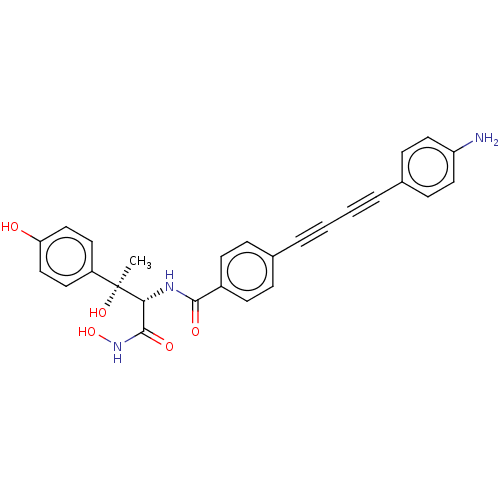 (CHEMBL2420203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50483252 (CHEMBL1643369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem 19: 852-60 (2011) Article DOI: 10.1016/j.bmc.2010.12.017 BindingDB Entry DOI: 10.7270/Q2DV1NQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50483252 (CHEMBL1643369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem 19: 852-60 (2011) Article DOI: 10.1016/j.bmc.2010.12.017 BindingDB Entry DOI: 10.7270/Q2DV1NQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM92267 (CS257) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem 19: 852-60 (2011) Article DOI: 10.1016/j.bmc.2010.12.017 BindingDB Entry DOI: 10.7270/Q2DV1NQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM92267 (CS257) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem 19: 852-60 (2011) Article DOI: 10.1016/j.bmc.2010.12.017 BindingDB Entry DOI: 10.7270/Q2DV1NQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50074960 ((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247630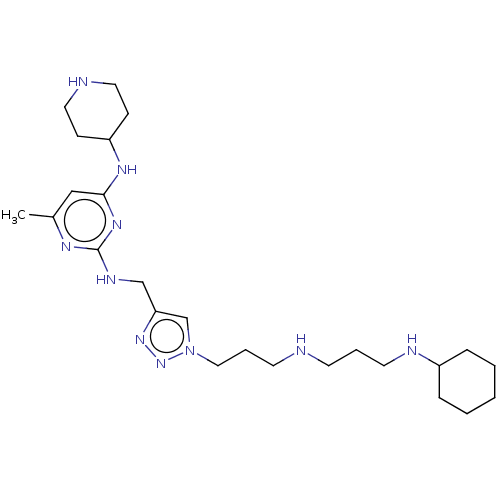 (CHEMBL4068500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247631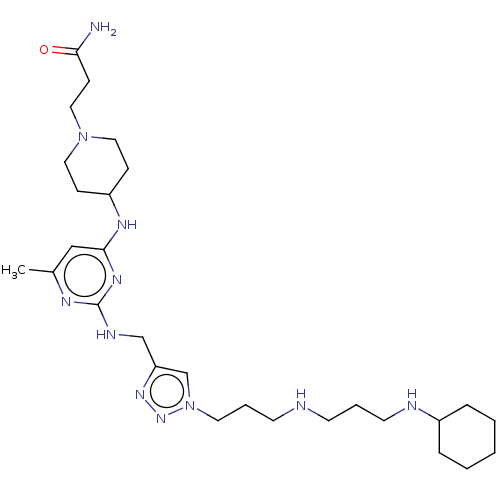 (CHEMBL4060426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50493222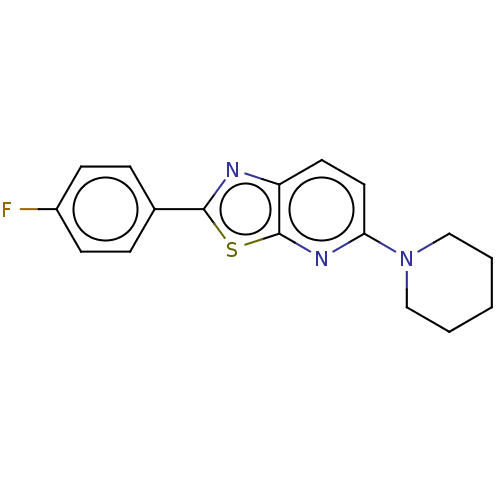 (CHEMBL2418211) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247595 (CHEMBL4064397) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247610 (CHEMBL4070263) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247609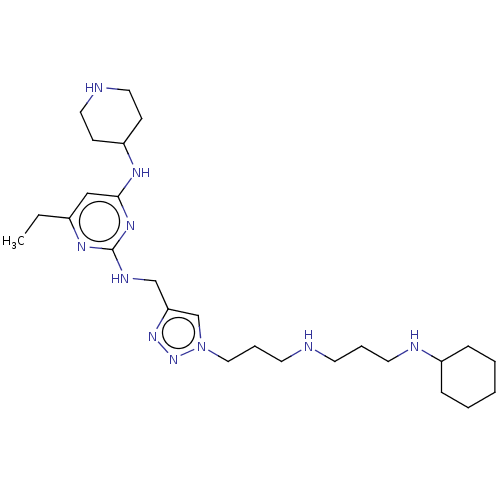 (CHEMBL4091201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247621 (CHEMBL4078499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247601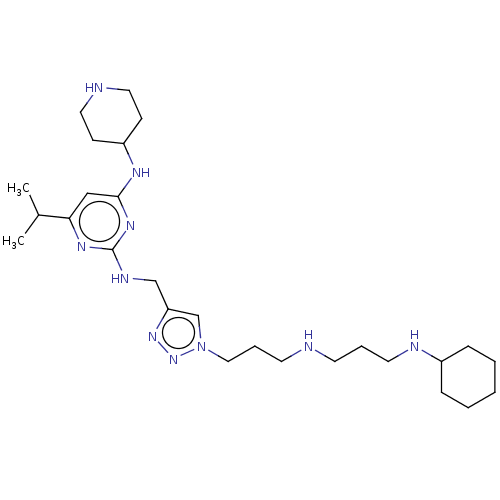 (CHEMBL4075454) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247617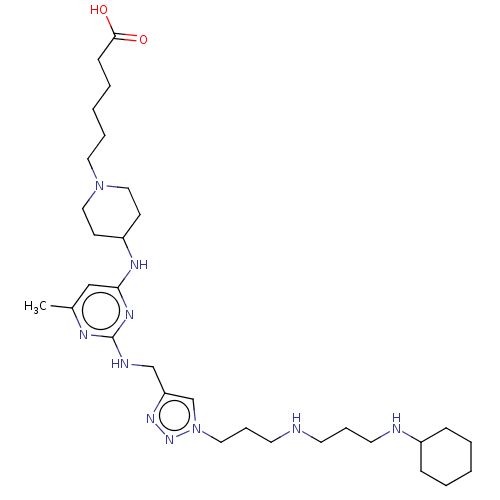 (CHEMBL4090175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247623 (CHEMBL4098404) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247626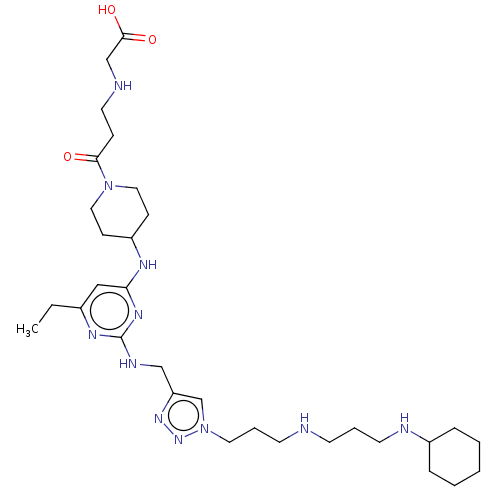 (CHEMBL4068647) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247627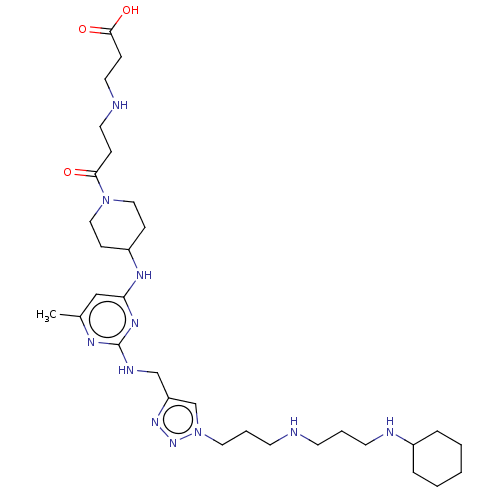 (CHEMBL4089763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247620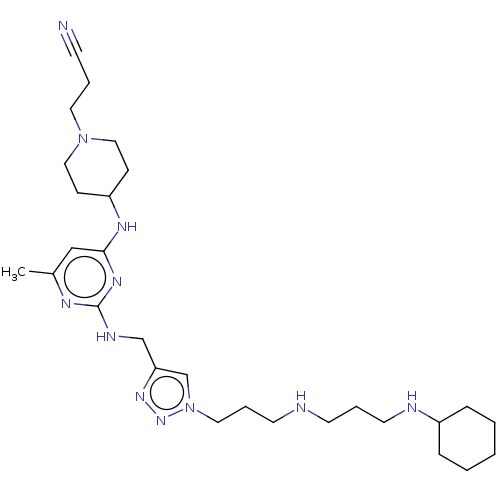 (CHEMBL4102780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247619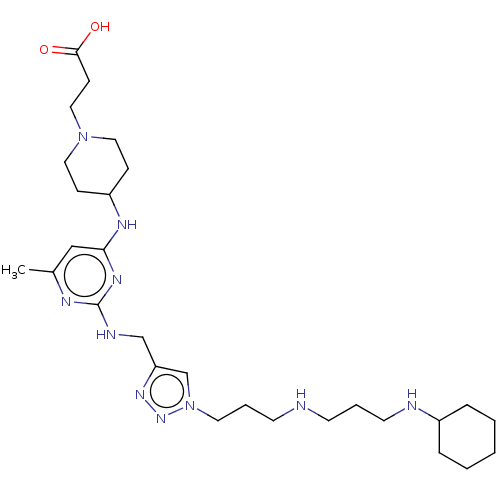 (CHEMBL4082328) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247594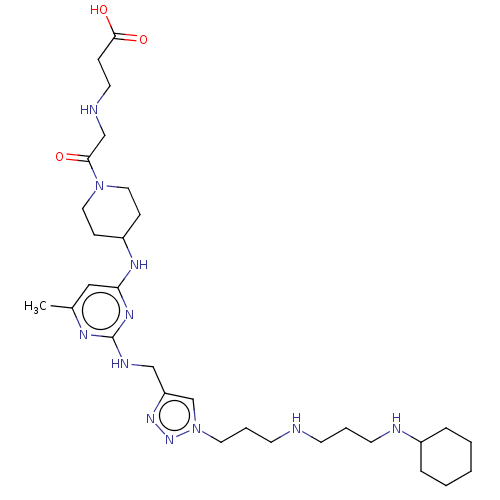 (CHEMBL4104222) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247593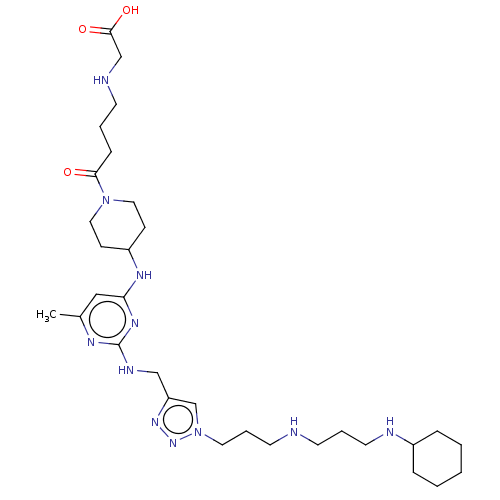 (CHEMBL4079981) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247632 (CHEMBL4081892) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247618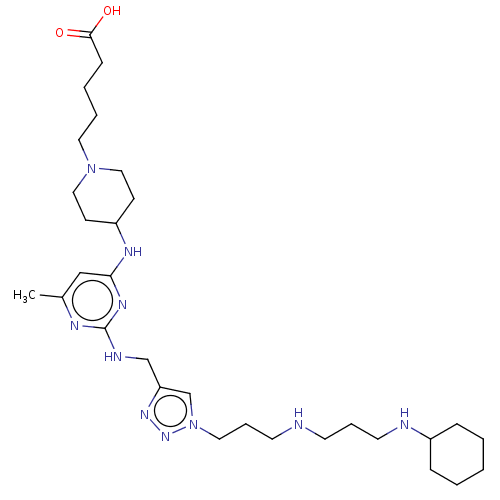 (CHEMBL4069110) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251807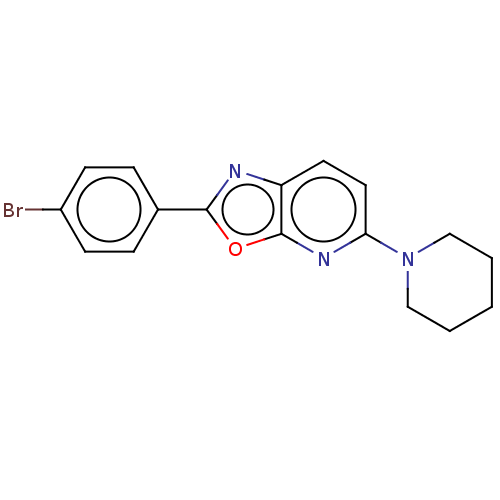 (US9469653, 5-47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description A stock solution was prepared using a human MAO-B enzyme (purchased from Aldrich) and a Amplex Red monoamine oxidase assay kit according to a prepara... | US Patent US9469653 (2016) BindingDB Entry DOI: 10.7270/Q2V9870D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247633 (CHEMBL4079460) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585229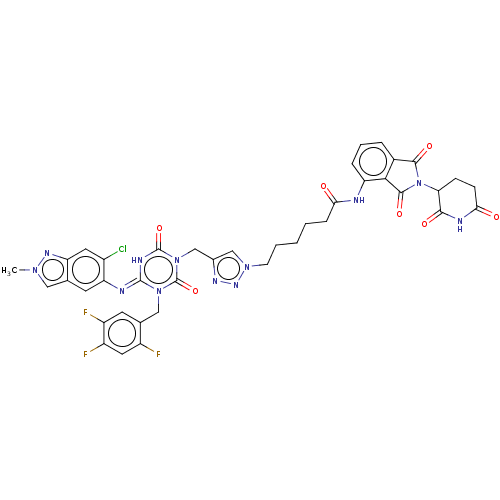 ((E)-6-(4-((4-((6-chloro-2-methyl-2H-indazol-5-yl)i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582035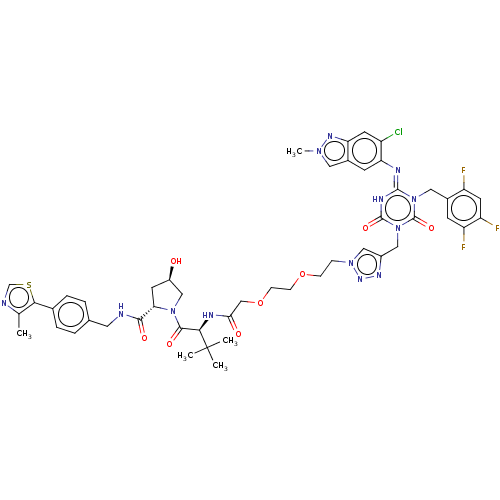 (Preparation of (2S,4R)-1-((S)-2-(2-(2-(2-(4-(((E)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582032 (Preparation of (2S,4R)-1-((S)-2-(7-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585233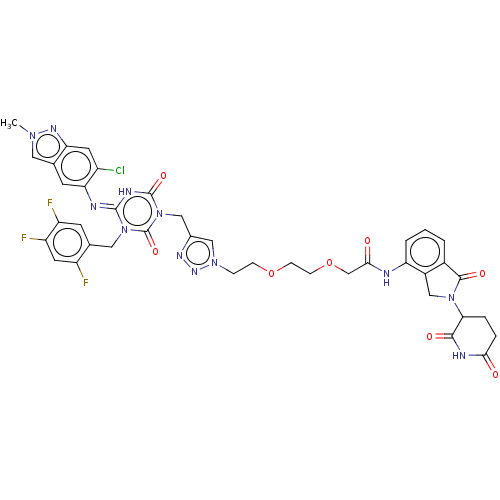 ((E)-2-(2-(2-(4-((4-((6-chloro-2-methyl-2H-indazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582033 (Preparation of (2S,4R)-1-((S)-2-(8-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585228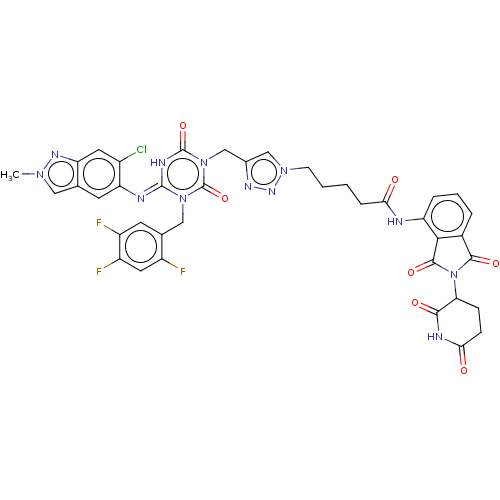 ((E)-5-(4-((4-((6-chloro-2-methyl-2H-indazol-5-yl)i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585227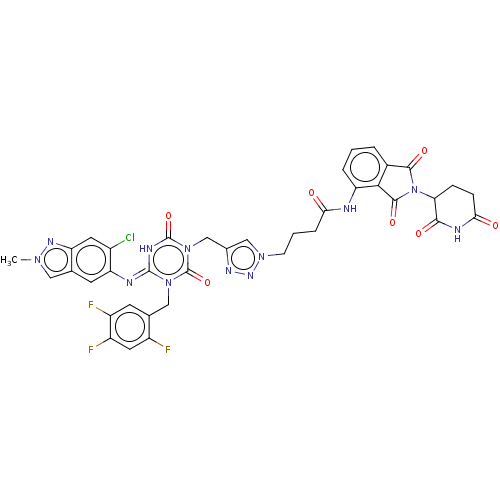 ((E)-4-(4-((4-((6-chloro-2-methyl-2H-indazol-5-yl)i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585137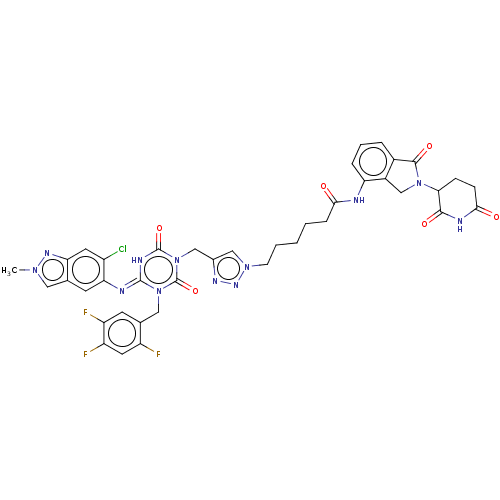 (US11530195, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585101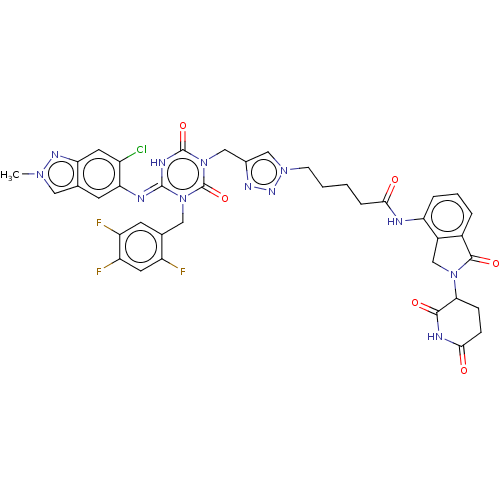 ((E)-5-(4-((4-((6-chloro-2-methyl-2H-indazol-5-yl)i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582031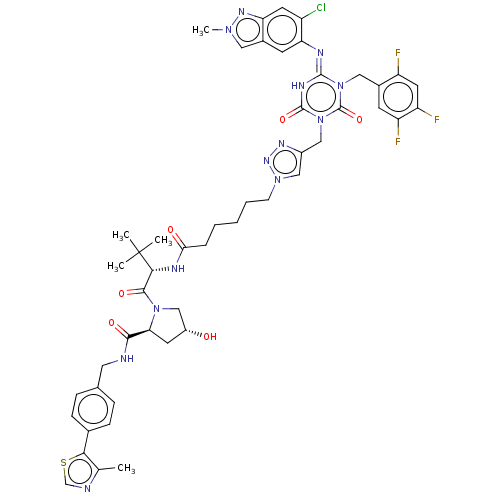 (Preparation of pyrrolidine-2-carboxamide of (2S,4R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM585236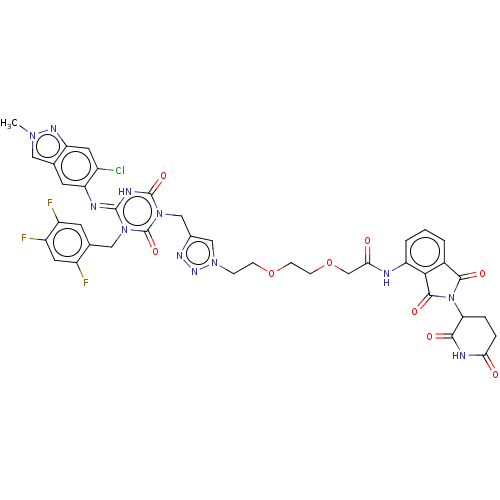 ((E)-2-(2-(2-(4-((4-((6-chloro-2-methyl-2H-indazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M61Q3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582036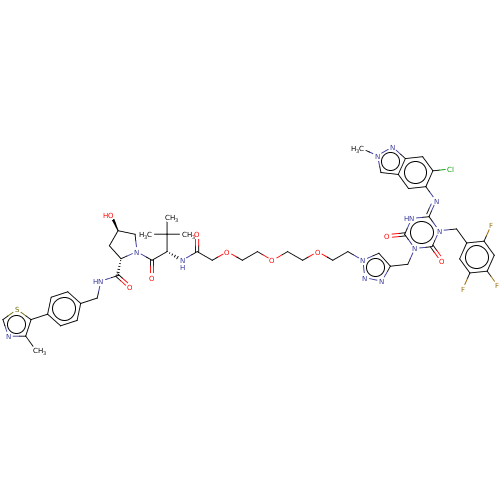 (Preparation of (2S,4R)-1-((S)-2-(tert-butyl)-14-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247622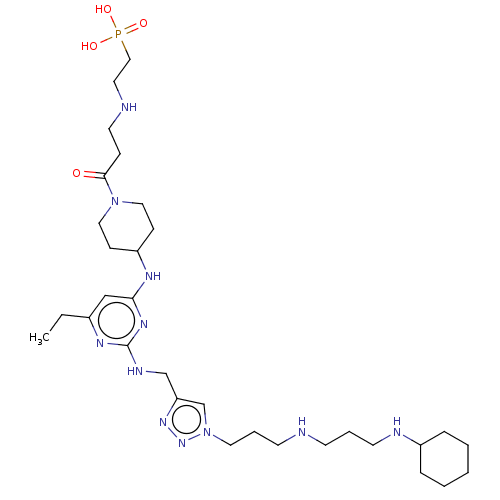 (CHEMBL4059987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247624 (CHEMBL4071355) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50493221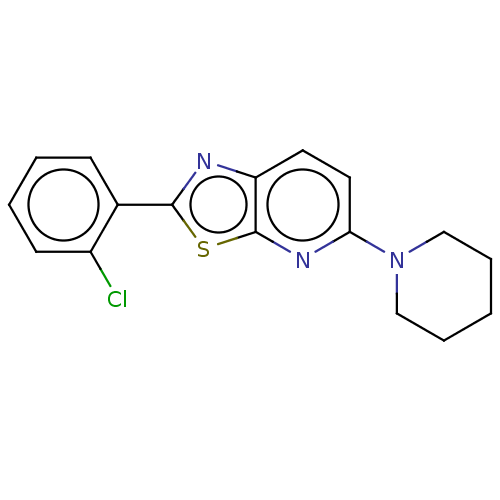 (CHEMBL2418212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696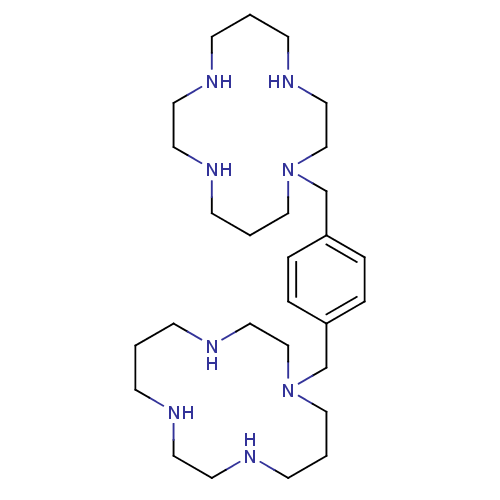 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | J Med Chem 61: 818-833 (2018) Article DOI: 10.1021/acs.jmedchem.7b01322 BindingDB Entry DOI: 10.7270/Q2KH0QRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251803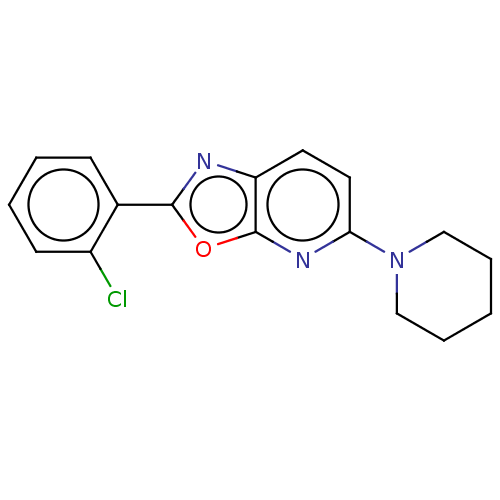 (US9469653, 5-39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251806 (US9469653, 5-45) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251804 (US9469653, 5-40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description A stock solution was prepared using a human MAO-B enzyme (purchased from Aldrich) and a Amplex Red monoamine oxidase assay kit according to a prepara... | US Patent US9469653 (2016) BindingDB Entry DOI: 10.7270/Q2V9870D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251804 (US9469653, 5-40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM251805 (US9469653, 5-41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay | Bioorg Med Chem 21: 5480-7 (2013) Article DOI: 10.1016/j.bmc.2013.05.066 BindingDB Entry DOI: 10.7270/Q2QN69PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |