

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13543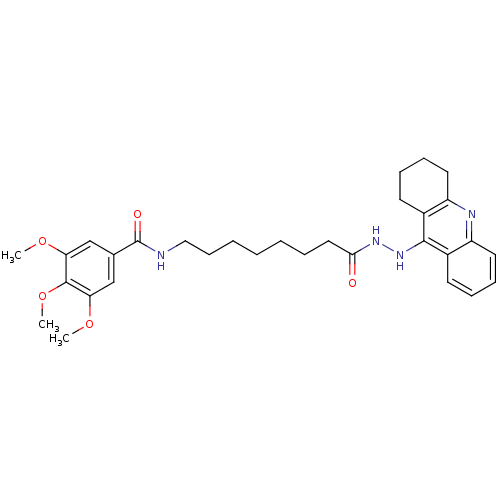 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.23 | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50244756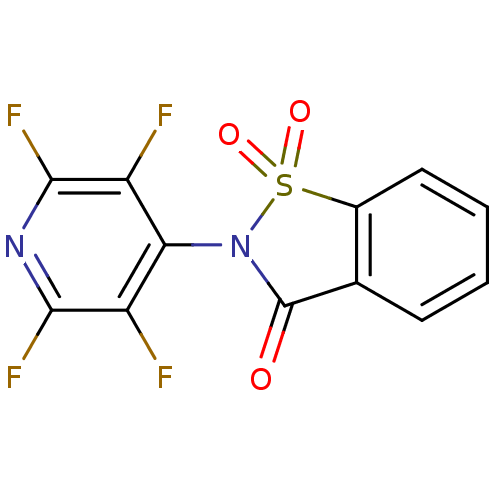 (2-(2,3,5,6-Tetrafluoropyridin-4-yl)-1,2-benzisothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 16: 8127-35 (2008) Article DOI: 10.1016/j.bmc.2008.07.049 BindingDB Entry DOI: 10.7270/Q26M36NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234353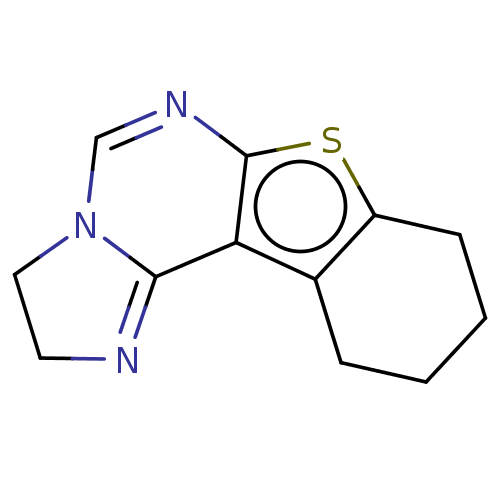 (2,3,8,9,10,11-Hexahydro-benzothieno[3,2-e]imidazo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | -33.6 | 1.75E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234356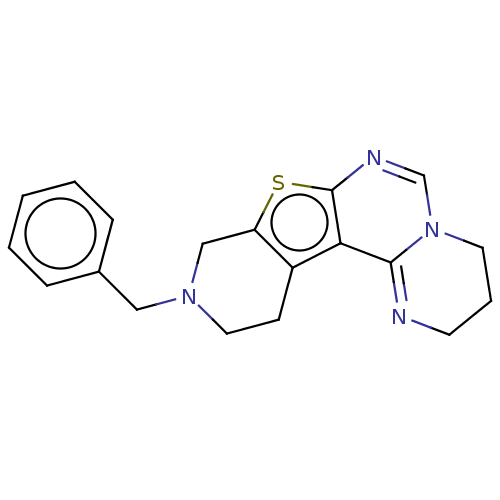 (10-Benzyl-3,4,9,10,11,12-hexahydro-2H-pyrido[4R...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | -33.2 | 1.73E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234351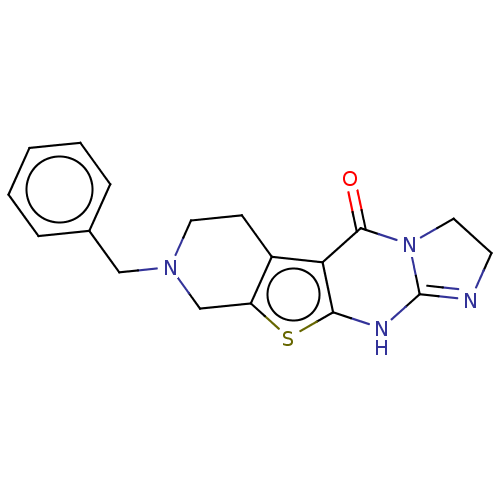 (8-Benzyl-2,3,6,7,8,9-hexahydro-imidazo[1,2-a]pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.55E+3 | -33.2 | 1.35E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234354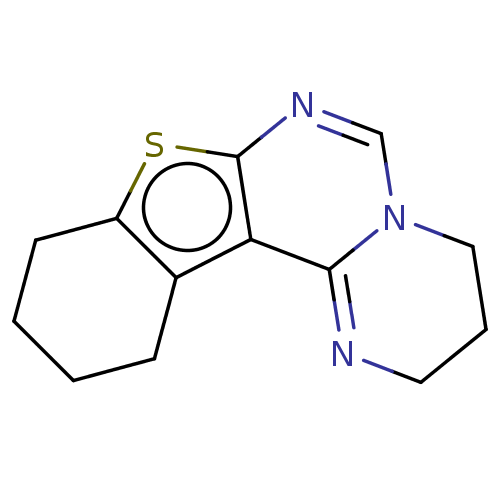 (3,4,9,10,11,12-Hexahydro-2H-benzothieno[3,2-e]pyri...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.92E+3 | -32.6 | 2.26E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234352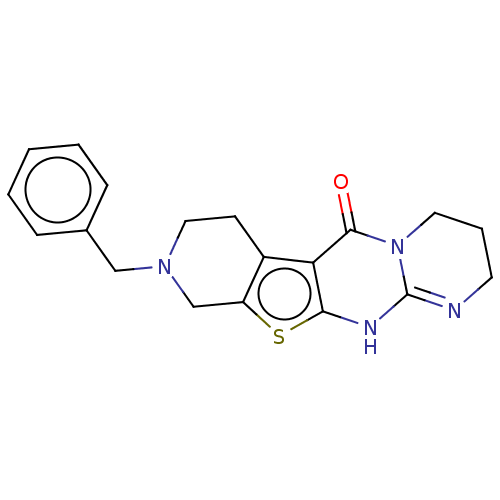 (9-Benzyl-2,3,4,7,8,9,10,12-octahydro-6H-pyrido[4&#...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.07E+3 | -32.4 | 1.51E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234355 (9-Benzyl-2,3,8,9,10,11-hexahydro-imidazo[1,2-c]pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.36E+3 | -32.1 | 3.09E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13549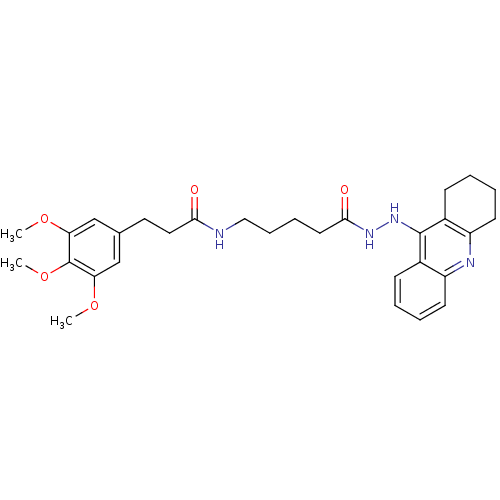 (3-(3,4,5-Trimethoxyphenyl)-N-(5-oxo-5-(2-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.139 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13550 (3-(3,4,5-Trimethoxyphenyl)-N-(6-oxo-6-(2-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13548 (3-(3,4,5-Trimethoxyphenyl)-N-(4-oxo-4-(2-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13543 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.293 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13542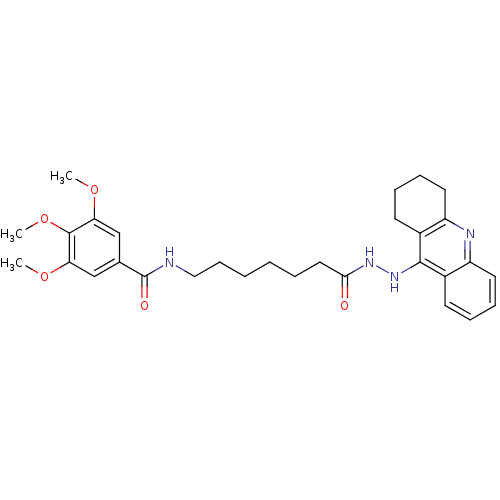 (3,4,5-Trimethoxy-N-(7-oxo-7-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.523 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13541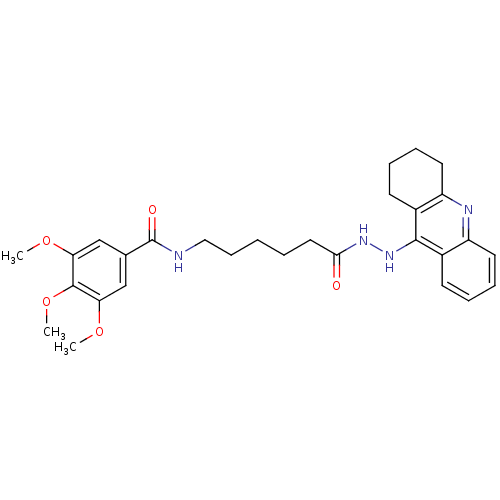 (3,4,5-Trimethoxy-N-(6-oxo-6-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.969 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13544 (3,4,5-Trimethoxy-N-(9-oxo-9-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13540 (3,4,5-Trimethoxy-N-(5-oxo-5-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13536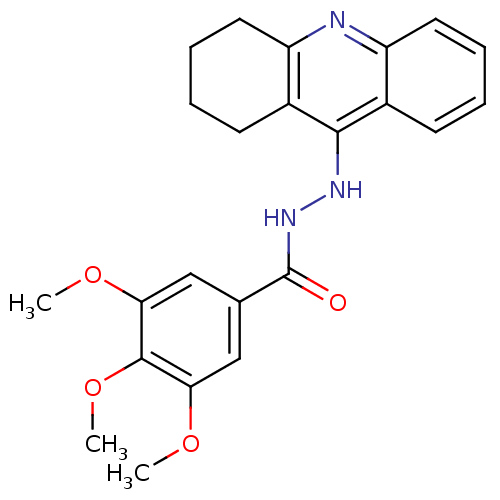 (3,4,5-trimethoxy-N'-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13544 (3,4,5-Trimethoxy-N-(9-oxo-9-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13544 (3,4,5-Trimethoxy-N-(9-oxo-9-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.08 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13543 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.24 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13542 (3,4,5-Trimethoxy-N-(7-oxo-7-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13544 (3,4,5-Trimethoxy-N-(9-oxo-9-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13547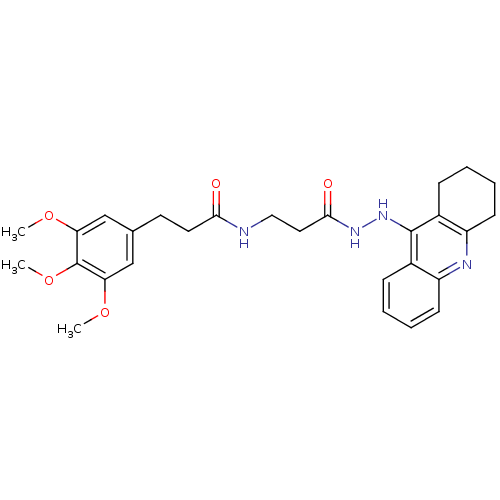 (3-(3,4,5-Trimethoxyphenyl)-N-(3-oxo-3-(2-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13539 (3,4,5-Trimethoxy-N-(4-oxo-4-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13550 (3-(3,4,5-Trimethoxyphenyl)-N-(6-oxo-6-(2-(1,2,3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.39 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13542 (3,4,5-Trimethoxy-N-(7-oxo-7-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13545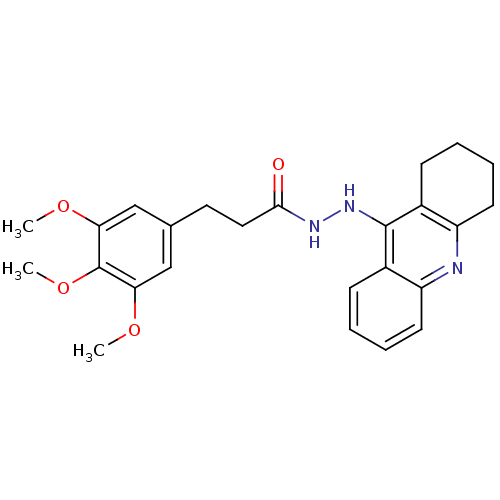 (N'-(1,2,3,4-tetrahydroacridin-9-yl)-3-(3,4,5-trime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13538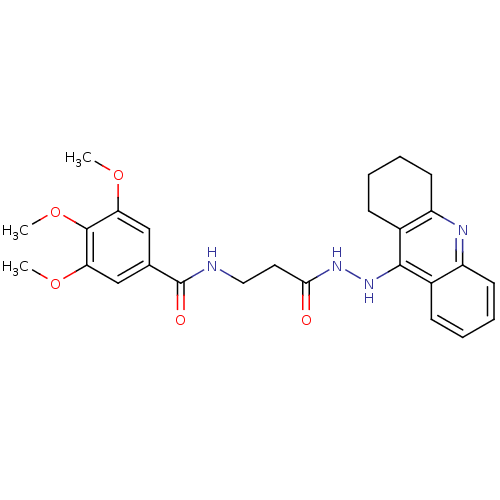 (3,4,5-Trimethoxy-N-(3-oxo-3-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13540 (3,4,5-Trimethoxy-N-(5-oxo-5-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13543 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13540 (3,4,5-Trimethoxy-N-(5-oxo-5-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13541 (3,4,5-Trimethoxy-N-(6-oxo-6-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13541 (3,4,5-Trimethoxy-N-(6-oxo-6-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13550 (3-(3,4,5-Trimethoxyphenyl)-N-(6-oxo-6-(2-(1,2,3,4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13540 (3,4,5-Trimethoxy-N-(5-oxo-5-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13536 (3,4,5-trimethoxy-N'-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13546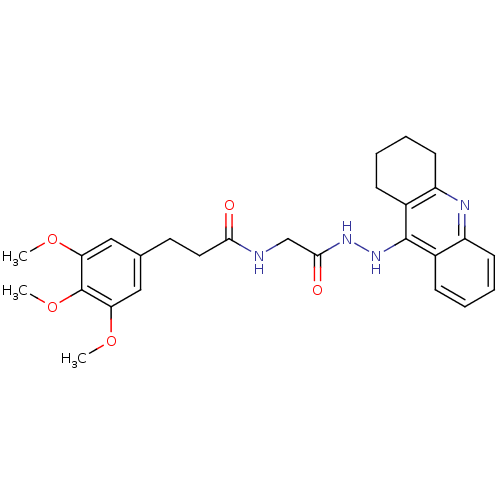 (3-(3,4,5-Trimethoxyphenyl)-N-(2-oxo-2-(2-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13542 (3,4,5-Trimethoxy-N-(7-oxo-7-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13539 (3,4,5-Trimethoxy-N-(4-oxo-4-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13537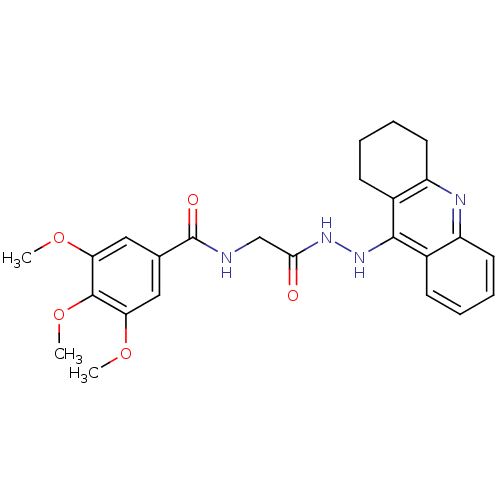 (3,4,5-Trimethoxy-N-(2-oxo-2-(2-(1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13550 (3-(3,4,5-Trimethoxyphenyl)-N-(6-oxo-6-(2-(1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13546 (3-(3,4,5-Trimethoxyphenyl)-N-(2-oxo-2-(2-(1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13545 (N'-(1,2,3,4-tetrahydroacridin-9-yl)-3-(3,4,5-trime...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13546 (3-(3,4,5-Trimethoxyphenyl)-N-(2-oxo-2-(2-(1,2,3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13538 (3,4,5-Trimethoxy-N-(3-oxo-3-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13537 (3,4,5-Trimethoxy-N-(2-oxo-2-(2-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.6 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13548 (3-(3,4,5-Trimethoxyphenyl)-N-(4-oxo-4-(2-(1,2,3,4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13549 (3-(3,4,5-Trimethoxyphenyl)-N-(5-oxo-5-(2-(1,2,3,4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM13541 (3,4,5-Trimethoxy-N-(6-oxo-6-(2-(1,2,3,4-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM13549 (3-(3,4,5-Trimethoxyphenyl)-N-(5-oxo-5-(2-(1,2,3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |