

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520437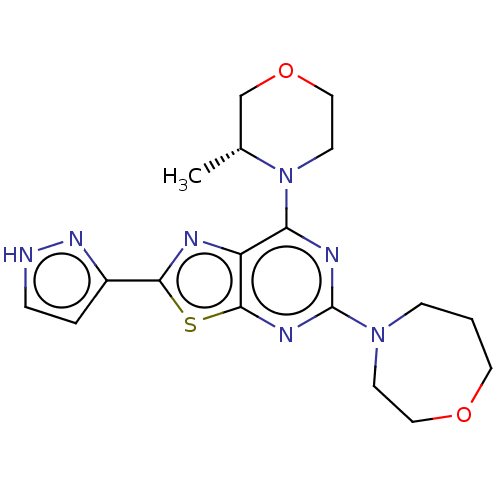 (CHEMBL4562597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520427 (CHEMBL4470066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit gamma-1 (Rattus norvegicus) | BDBM50021185 (5-Aminomethyl-4,5-dihydro-isoxazol-3-ol | CHEMBL42...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GABA binding to Gamma-aminobutyric-acid receptor of rat brain synaptic membranes | J Med Chem 28: 1612-7 (1985) BindingDB Entry DOI: 10.7270/Q2PN94NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520433 (CHEMBL4451234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520428 (CHEMBL4518369) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit gamma-1 (Rattus norvegicus) | BDBM23183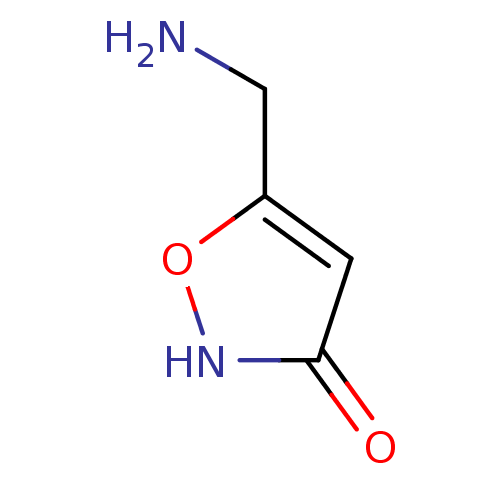 (5-(aminomethyl)-1,2-oxazol-3-ol | Agarin | CHEMBL2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GABA binding to Gamma-aminobutyric-acid receptor of rat brain synaptic membranes | J Med Chem 28: 1612-7 (1985) BindingDB Entry DOI: 10.7270/Q2PN94NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520435 (CHEMBL4442382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520430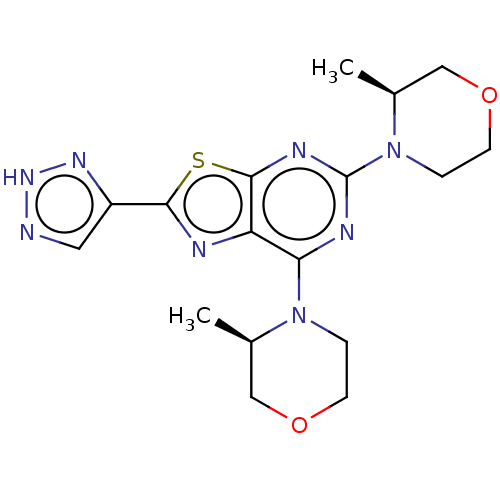 (CHEMBL4515125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520431 (CHEMBL4566380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR L1460P mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR S2215Y mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot analysis (Rvb = 19... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR S2215F mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50520428 (CHEMBL4518369) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) assessed as reduction in CHK2 phosphorylation by cell based assay | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR E2419K mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR L2427P mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit gamma-1 (Rattus norvegicus) | BDBM24183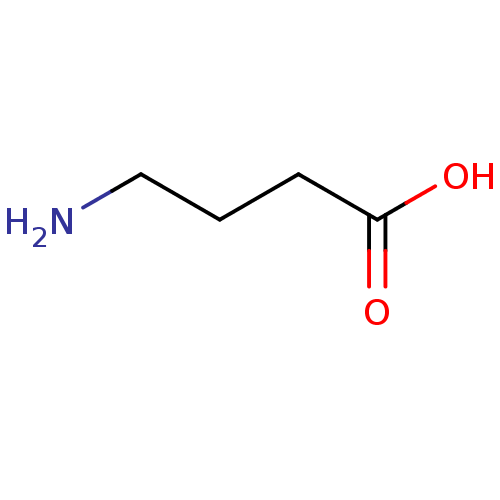 (4-amino-n-[2,3-3H]butyric acid | 4-aminobutanoic a...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GABA binding to Gamma-aminobutyric-acid receptor of rat brain synaptic membranes | J Med Chem 28: 1612-7 (1985) BindingDB Entry DOI: 10.7270/Q2PN94NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Rattus norvegicus) | BDBM50520428 (CHEMBL4518369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR C1483Y mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520426 (CHEMBL4514031) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse TSC1-/- MEF cells assessed as inhibition of S6 Ser240/244 phosphorylation incubated for 2 hrs by fluorescence based assay | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520438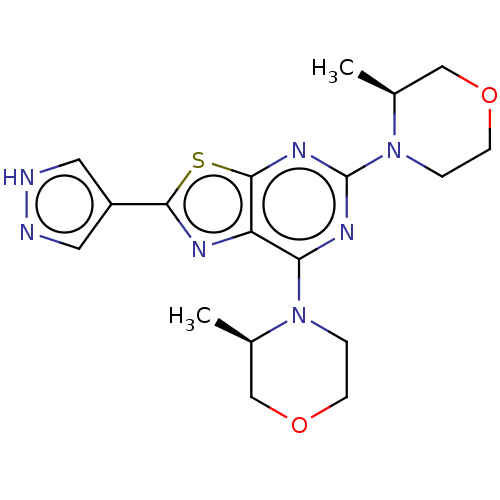 (CHEMBL4460774) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465930 (CHEMBL4292990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520439 (CHEMBL4468727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465915 (CHEMBL4281315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520434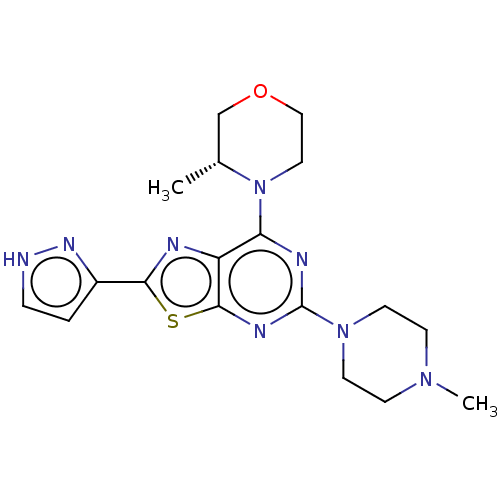 (CHEMBL4522946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022273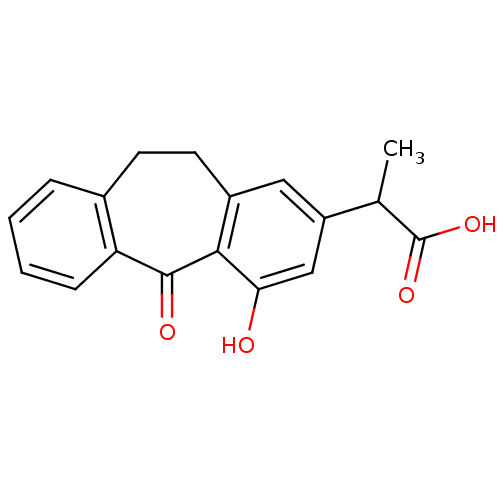 (2-(4-Hydroxy-5-oxo-10,11-dihydro-5H-dibenzo[a,d]cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50520428 (CHEMBL4518369) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PDE4D (unknown origin) | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465921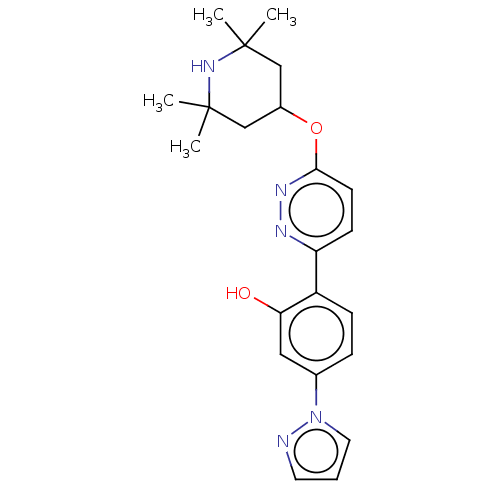 (CHEMBL4288439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit gamma-1 (Rattus norvegicus) | BDBM50021189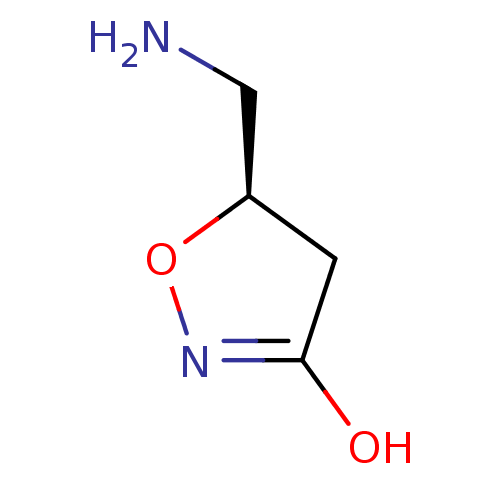 (5-Aminomethyl-4,5-dihydro-isoxazol-3-ol | CHEMBL40...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GABA binding to Gamma-aminobutyric-acid receptor of rat brain synaptic membranes | J Med Chem 28: 1612-7 (1985) BindingDB Entry DOI: 10.7270/Q2PN94NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465926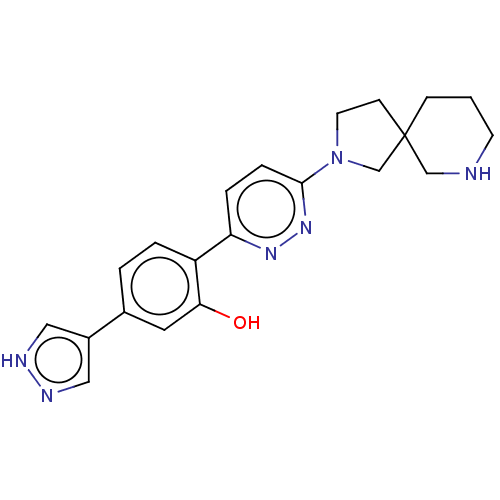 (CHEMBL4294614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520436 (CHEMBL4435029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465919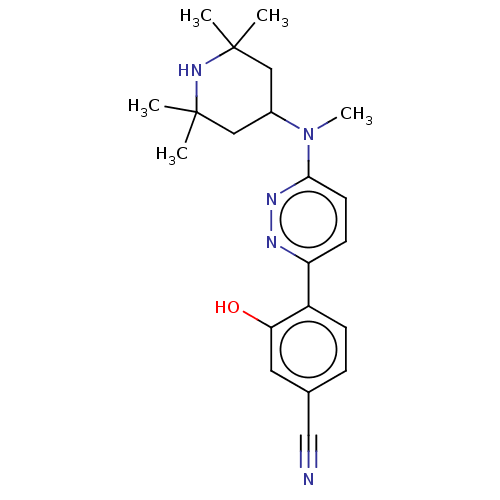 (CHEMBL4283397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465920 (CHEMBL4280558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520429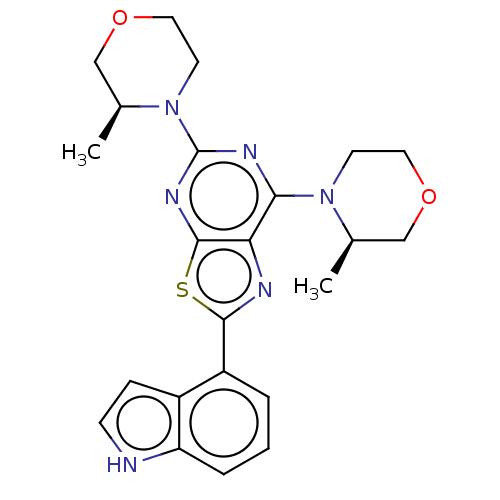 (CHEMBL4574882) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 305 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022265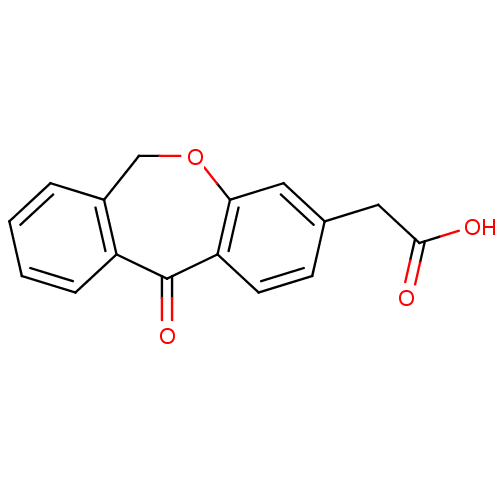 ((11-Oxo-6,11-dihydro-dibenzo[b,e]oxepin-3-yl)-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50520428 (CHEMBL4518369) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) assessed as reduction in CHK1 phosphorylation by cell based assay | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit gamma-1 (Rattus norvegicus) | BDBM50021186 (4-Amino-3-hydroxy-butyric acid | CHEMBL296263 | S(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GABA binding to Gamma-aminobutyric-acid receptor of rat brain synaptic membranes | J Med Chem 28: 1612-7 (1985) BindingDB Entry DOI: 10.7270/Q2PN94NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465916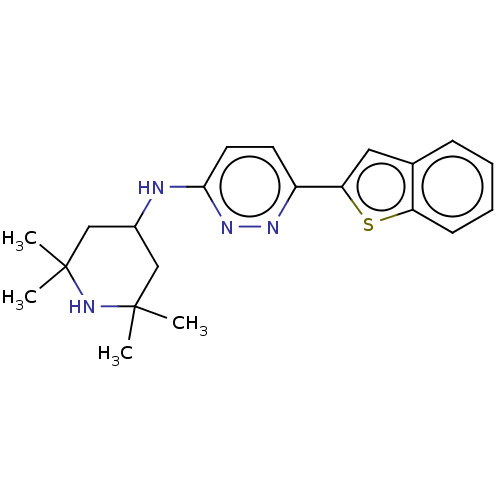 (CHEMBL4289183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Mus musculus (Mouse)) | BDBM50520432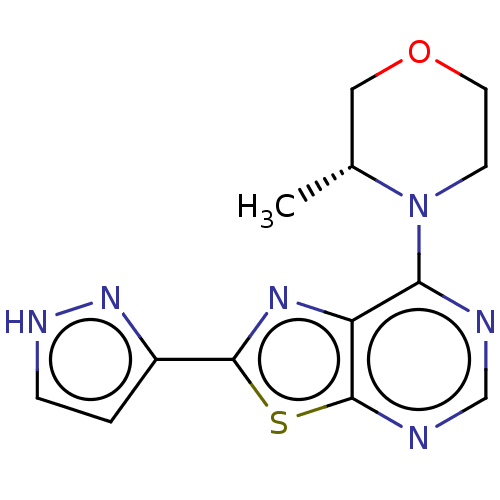 (CHEMBL4538499) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022274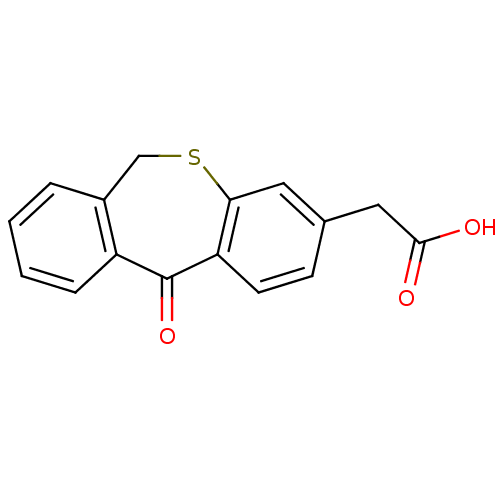 ((5-Oxo-5,11-dihydro-10-thia-dibenzo[a,d]cyclohepte...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022263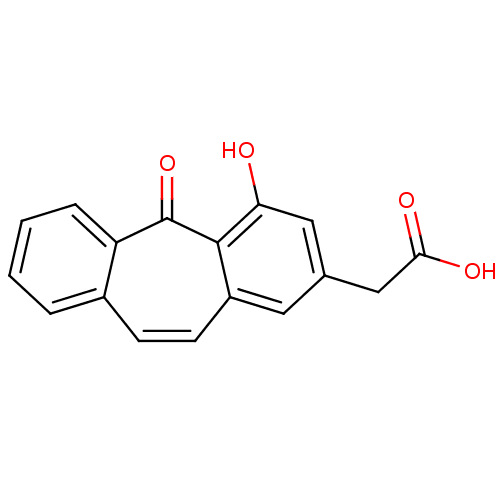 ((4-Hydroxy-5-oxo-5H-dibenzo[a,d]cyclohepten-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022260 (11,12-Dihydro-3H-1-oxa-benzo[4,5]cyclohepta[1,2-e]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50520426 (CHEMBL4514031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022264 ((7-Hydroxy-5-oxo-10,11-dihydro-5H-dibenzo[a,d]cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50022268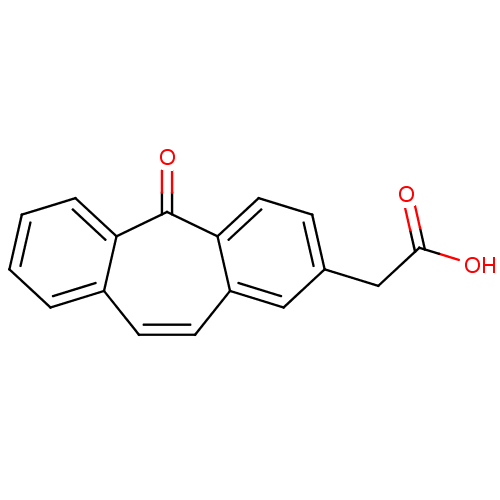 ((5-Oxo-5H-dibenzo[a,d]cyclohepten-2-yl)-acetic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lens aldose reductase. | J Med Chem 29: 2347-51 (1986) BindingDB Entry DOI: 10.7270/Q23R0RWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50520428 (CHEMBL4518369) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50465927 (CHEMBL4285798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting | J Med Chem 61: 11021-11036 (2018) Article DOI: 10.1021/acs.jmedchem.8b01291 BindingDB Entry DOI: 10.7270/Q2474DJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50520428 (CHEMBL4518369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50520426 (CHEMBL4514031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 63: 1068-1083 (2020) Article DOI: 10.1021/acs.jmedchem.9b01398 BindingDB Entry DOI: 10.7270/Q29S1VFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent betaine transporter (Rattus norvegicus) | BDBM50226144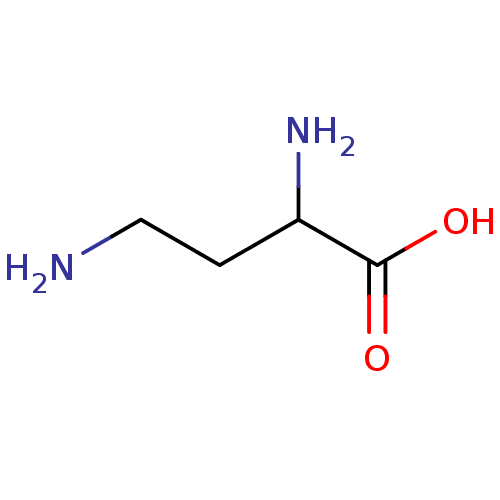 (2,4-Diamino-Butyric Acid | CHEBI:64307 | CHEMBL307...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal (synaptosomal) GABA uptake. | J Med Chem 29: 224-9 (1986) BindingDB Entry DOI: 10.7270/Q2FX7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104 total ) | Next | Last >> |