

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673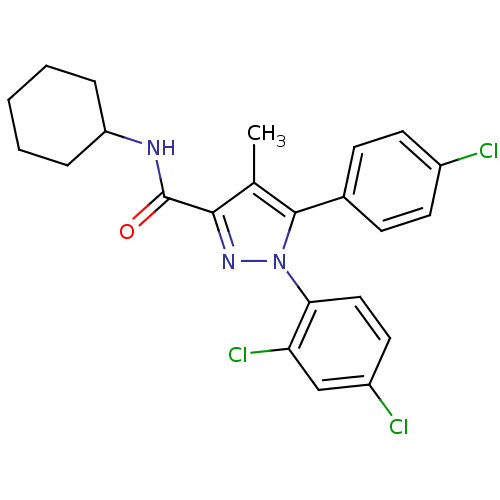 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with wild type Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50117177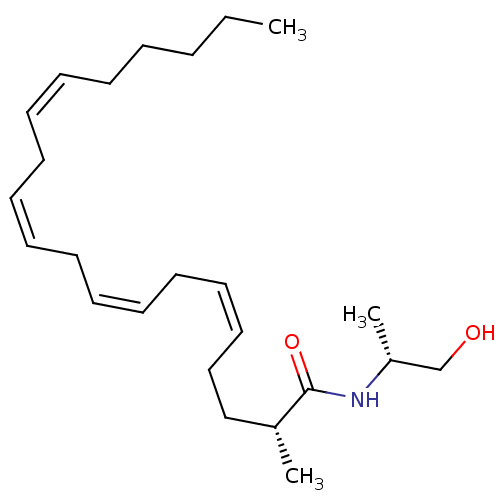 (1N-[2-hydroxy-1-methyl-(1R)-ethyl]-2-methyl-(2R,5Z...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.25 (190)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with wild type Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.36 (201)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493249 (CHEMBL2420249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.25 (190)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493245 (CHEMBL2420251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224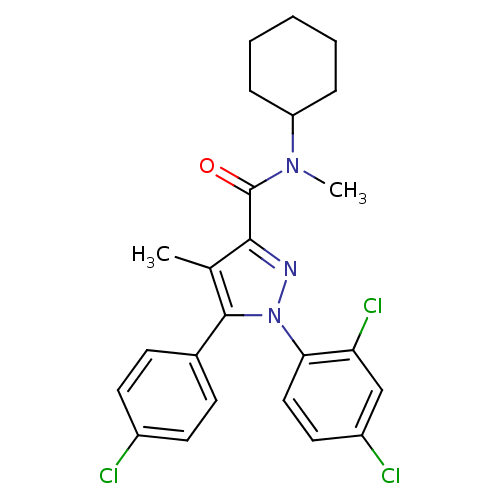 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530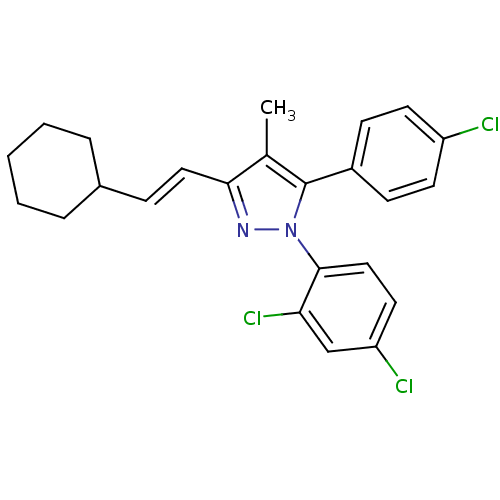 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with W6 48(357)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM85675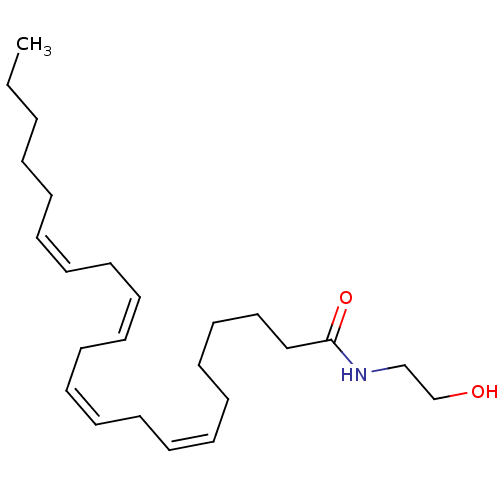 (Anandamide + PMSF | CHEMBL321585) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 34.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 39.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with W6 48(357)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056457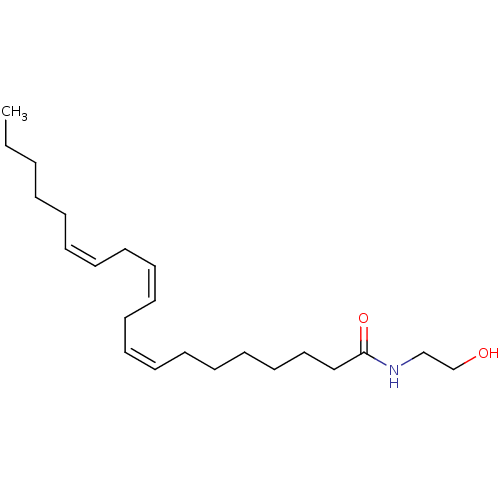 ((8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-8,11,14-trien...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 53.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493248 (CHEMBL2420252) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.36 (201)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50117178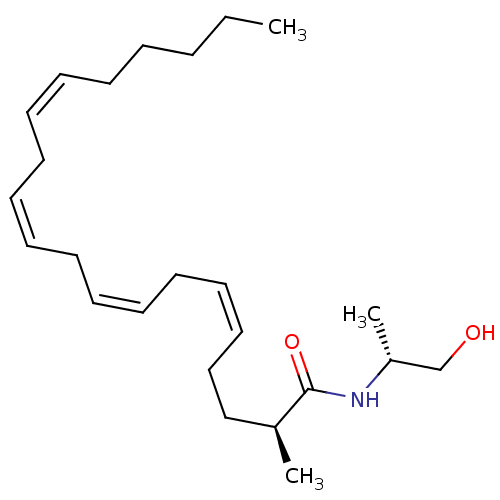 (1N-[2-hydroxy-1-methyl-(1R)-ethyl]-2-methyl-(2S,5Z...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with W5 43(280)A mutant Cannabinoid receptor 1 at 5... | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50117180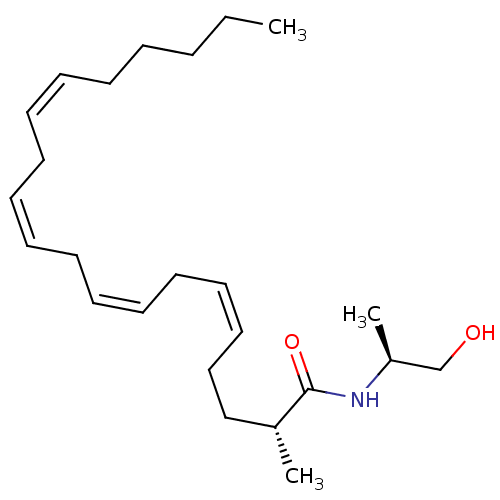 (1N-[2-hydroxy-1-methyl-(1S)-ethyl]-2-methyl-(2R,5Z...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with wild type Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.36 (201)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with W6 48(357)A mutant in Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with W5 43(280)A mutant Cannabinoid receptor 1 at 5... | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50117179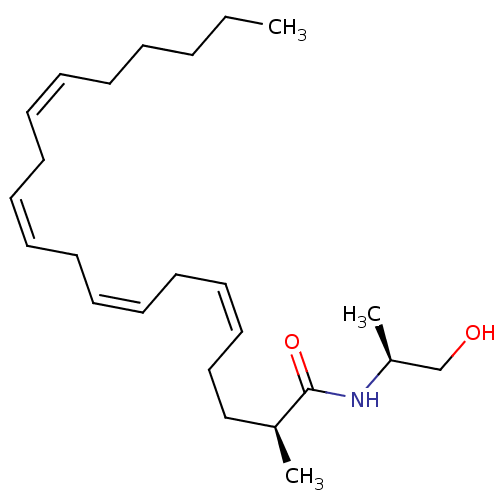 (1N-[2-hydroxy-1-methyl-(1S)-ethyl]-2-methyl-(2S,5Z...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493247 (CHEMBL2420248) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 527 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493246 (CHEMBL2420250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056456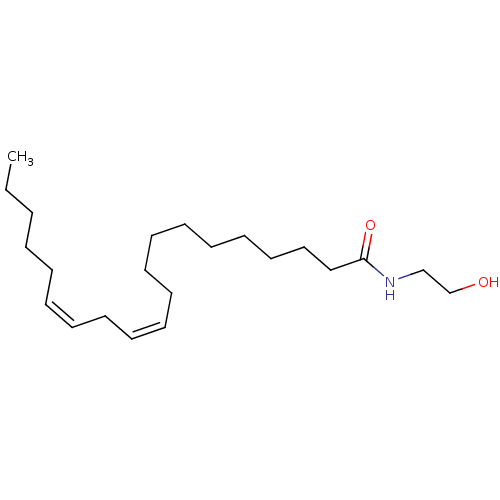 ((11Z,14Z)-Icosa-11,14-dienoic acid (2-hydroxy-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Cannabinoid receptor 1 [Inactive form(R) of CB1 receptor] | J Med Chem 45: 3649-59 (2002) BindingDB Entry DOI: 10.7270/Q2DB815G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forbes Norris ALS/MDA Research Center Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-CP-55,940 from the membranes prepared from HEK cell line with F3.25 (190)A mutant Cannabinoid receptor 1 | J Med Chem 46: 5139-52 (2003) Article DOI: 10.1021/jm0302647 BindingDB Entry DOI: 10.7270/Q28C9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493246 (CHEMBL2420250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Antagonist activity at CB2 receptor in HEK293 cells assessed as increase in CP-55,940 EC50 measuring inhibition of forskolin-stimulated cAMP accumula... | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493249 (CHEMBL2420249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Antagonist activity at CB2 receptor in HEK293 cells assessed as increase in CP-55,940 EC50 measuring inhibition of forskolin-stimulated cAMP accumula... | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493245 (CHEMBL2420251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Antagonist activity at CB2 receptor in HEK293 cells assessed as increase in CP-55,940 EC50 measuring inhibition of forskolin-stimulated cAMP accumula... | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Antagonist activity at CB2 receptor in HEK293 cells assessed as increase in CP-55,940 EC50 measuring inhibition of forskolin-stimulated cAMP accumula... | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |