 Found 11109 hits with Last Name = 'min' and Initial = 'd'
Found 11109 hits with Last Name = 'min' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50266025
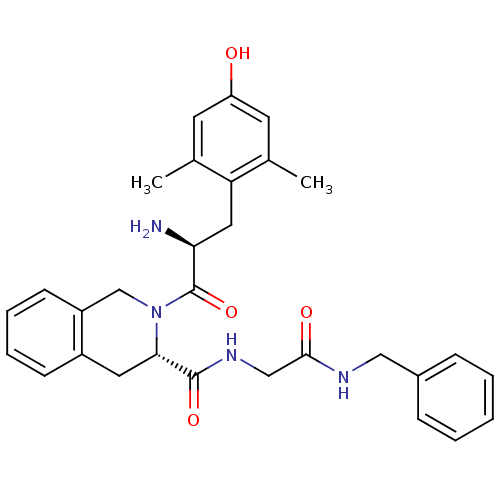
((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H34N4O4/c1-19-12-24(35)13-20(2)25(19)15-26(31)30(38)34-18-23-11-7-6-10-22(23)14-27(34)29(37)33-17-28(36)32-16-21-8-4-3-5-9-21/h3-13,26-27,35H,14-18,31H2,1-2H3,(H,32,36)(H,33,37)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes |
Bioorg Med Chem Lett 19: 433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.051
BindingDB Entry DOI: 10.7270/Q2K64HZ7 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948
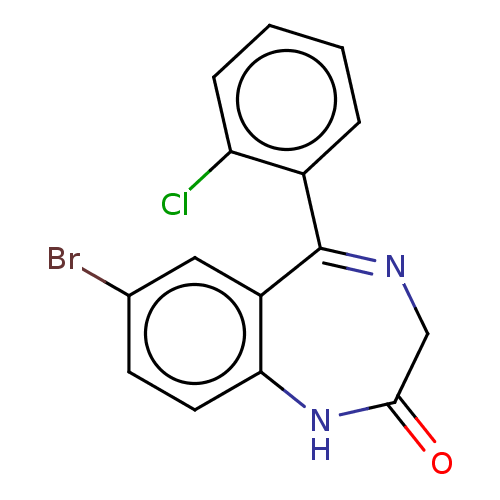
(PHENAZEPAM) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042715
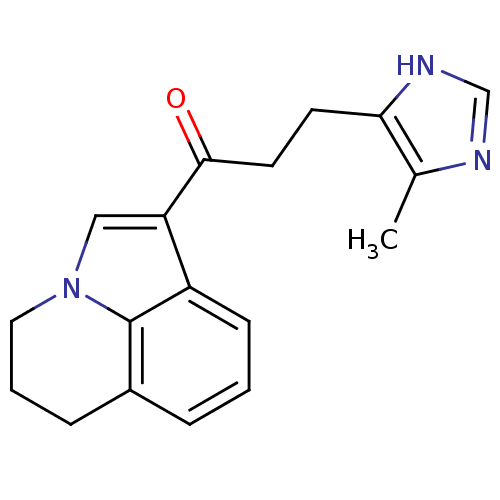
(1-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-...)Show InChI InChI=1S/C18H19N3O/c1-12-16(20-11-19-12)7-8-17(22)15-10-21-9-3-5-13-4-2-6-14(15)18(13)21/h2,4,6,10-11H,3,5,7-9H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
The binding affinity of the compound was measured on histamine H1 receptor using [3H]- mepyramine as radioligand. |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416
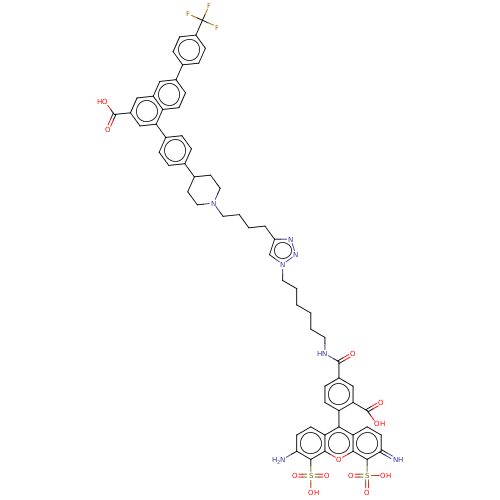
(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000
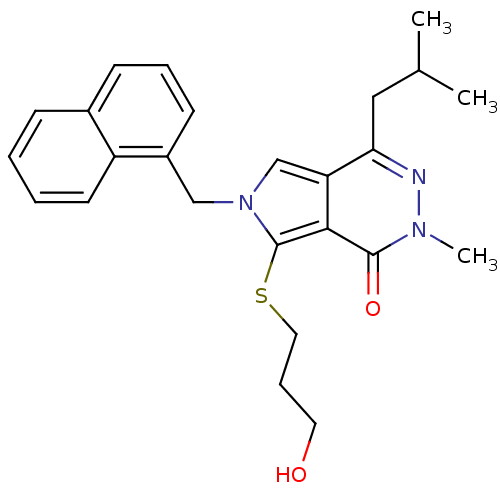
(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50308250
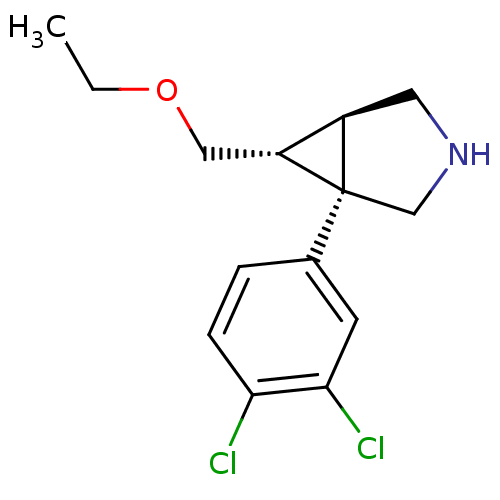
((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949
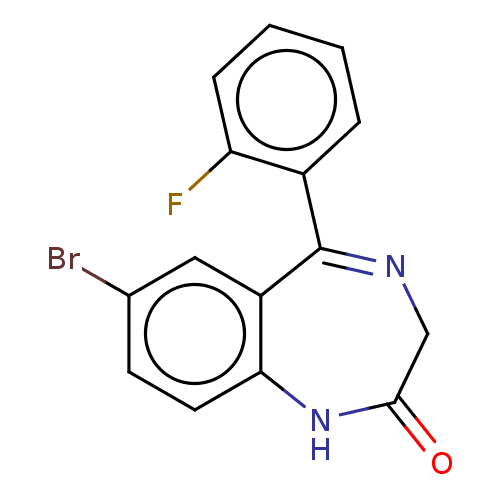
(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50266025

((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H34N4O4/c1-19-12-24(35)13-20(2)25(19)15-26(31)30(38)34-18-23-11-7-6-10-22(23)14-27(34)29(37)33-17-28(36)32-16-21-8-4-3-5-9-21/h3-13,26-27,35H,14-18,31H2,1-2H3,(H,32,36)(H,33,37)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes |
Bioorg Med Chem Lett 19: 433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.051
BindingDB Entry DOI: 10.7270/Q2K64HZ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042718
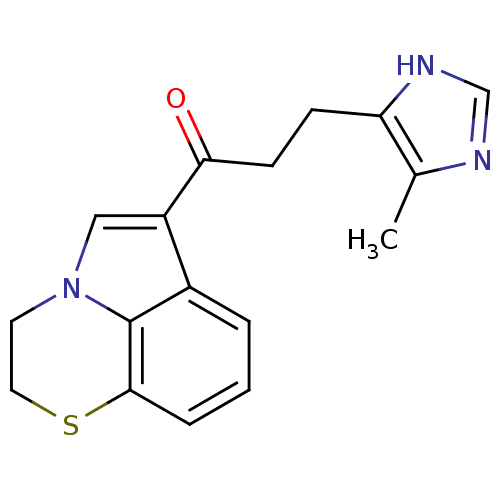
(1-(3,4-Dihydro-5-thia-2a-aza-acenaphthylen-1-yl)-3...)Show InChI InChI=1S/C17H17N3OS/c1-11-14(19-10-18-11)5-6-15(21)13-9-20-7-8-22-16-4-2-3-12(13)17(16)20/h2-4,9-10H,5-8H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
The binding affinity of the compound was measured on alpha2-adrenergic receptor using [3H]- clonidine as radioligand. |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50231569
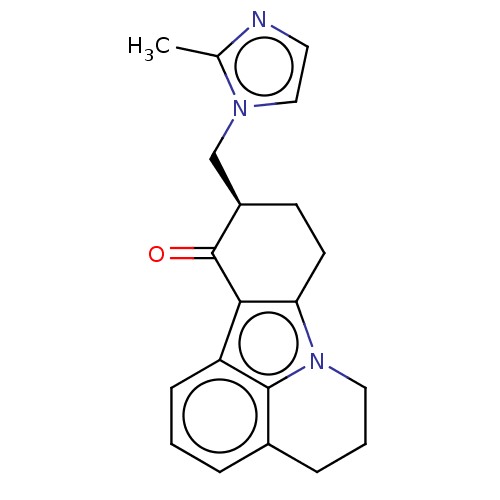
(Cilansetron | KC-9946)Show InChI InChI=1S/C20H21N3O/c1-13-21-9-11-22(13)12-15-7-8-17-18(20(15)24)16-6-2-4-14-5-3-10-23(17)19(14)16/h2,4,6,9,11,15H,3,5,7-8,10,12H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
The binding affinity was measured on 5-hydroxytryptamine 3 receptor using [3H]GR-65630 as radioligand. |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042714
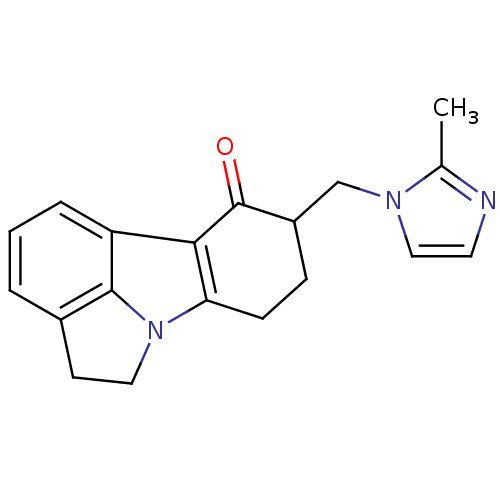
(9-(2-Methyl-imidazol-1-ylmethyl)-4,5,8,9-tetrahydr...)Show InChI InChI=1S/C19H19N3O/c1-12-20-8-10-21(12)11-14-5-6-16-17(19(14)23)15-4-2-3-13-7-9-22(16)18(13)15/h2-4,8,10,14H,5-7,9,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor in rat brain cortical membranes |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042712
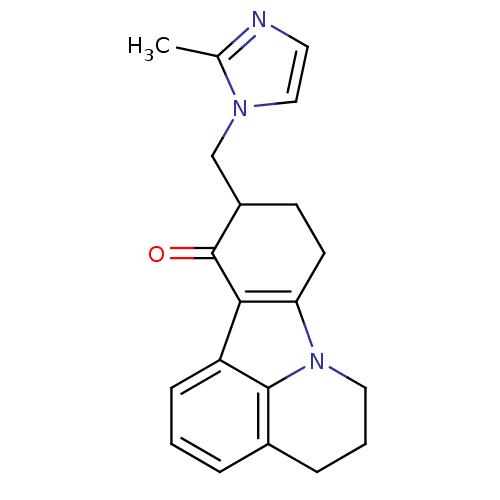
(10-(2-Methyl-imidazol-1-ylmethyl)-5,6,9,10-tetrahy...)Show InChI InChI=1S/C20H21N3O/c1-13-21-9-11-22(13)12-15-7-8-17-18(20(15)24)16-6-2-4-14-5-3-10-23(17)19(14)16/h2,4,6,9,11,15H,3,5,7-8,10,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]- GR-65,630 binding to 5-hydroxytryptamine 3 receptor in rat brain cortical membranes |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50084650
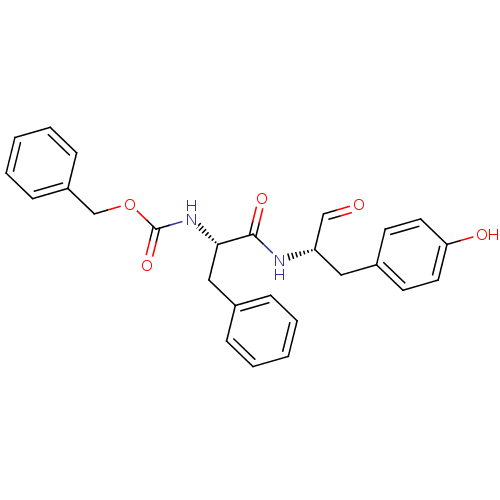
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001
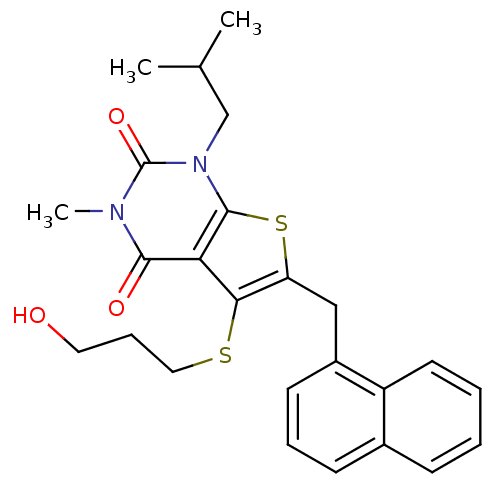
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042713
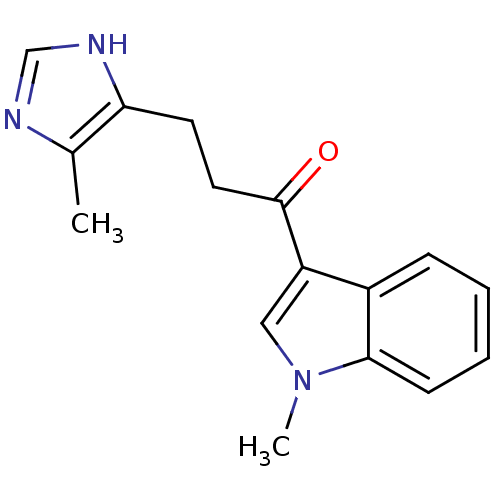
(3-(5-Methyl-1H-imidazol-4-yl)-1-(1-methyl-1H-indol...)Show InChI InChI=1S/C16H17N3O/c1-11-14(18-10-17-11)7-8-16(20)13-9-19(2)15-6-4-3-5-12(13)15/h3-6,9-10H,7-8H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
The binding affinity of the compound was measured on glycine receptor using [3H]- strychnine as radioligand. |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266666
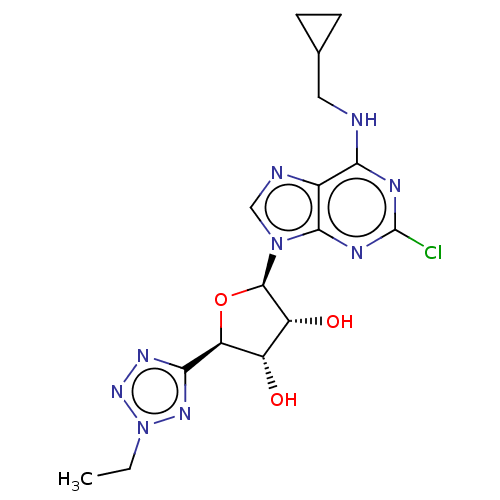
(CHEMBL4105164)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC3CC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H20ClN9O3/c1-2-26-23-13(22-24-26)11-9(27)10(28)15(29-11)25-6-19-8-12(18-5-7-3-4-7)20-16(17)21-14(8)25/h6-7,9-11,15,27-28H,2-5H2,1H3,(H,18,20,21)/t9-,10+,11-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986
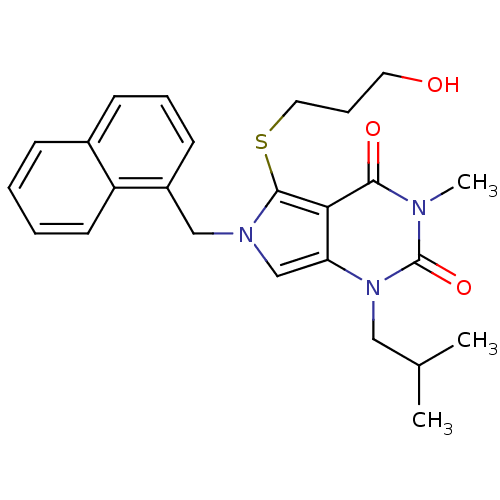
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50042720
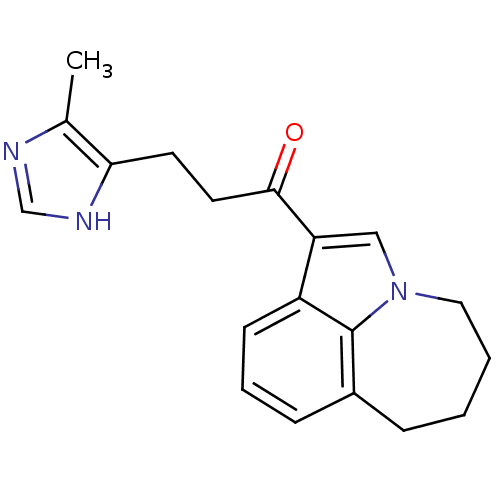
(3-(5-Methyl-1H-imidazol-4-yl)-1-(4,5,6,7-tetrahydr...)Show InChI InChI=1S/C19H21N3O/c1-13-17(21-12-20-13)8-9-18(23)16-11-22-10-3-2-5-14-6-4-7-15(16)19(14)22/h4,6-7,11-12H,2-3,5,8-10H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar B.V.
Curated by ChEMBL
| Assay Description
The binding affinity of the compound was measured on muscarine M1 receptor using [3H]- pirenzepine as radioligand. |
J Med Chem 36: 3693-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NS0SZH |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266664
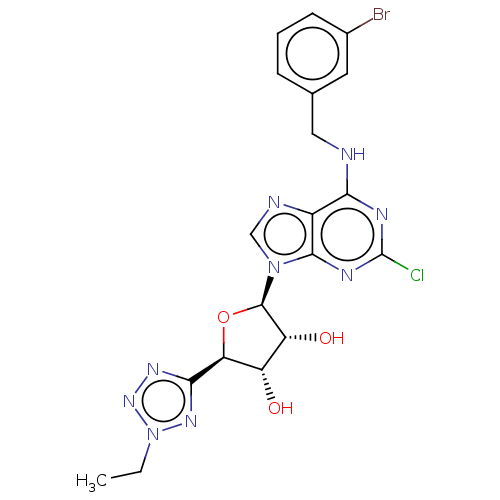
(CHEMBL4059961)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Br)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H19BrClN9O3/c1-2-30-27-16(26-28-30)14-12(31)13(32)18(33-14)29-8-23-11-15(24-19(21)25-17(11)29)22-7-9-4-3-5-10(20)6-9/h3-6,8,12-14,18,31-32H,2,7H2,1H3,(H,22,24,25)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002
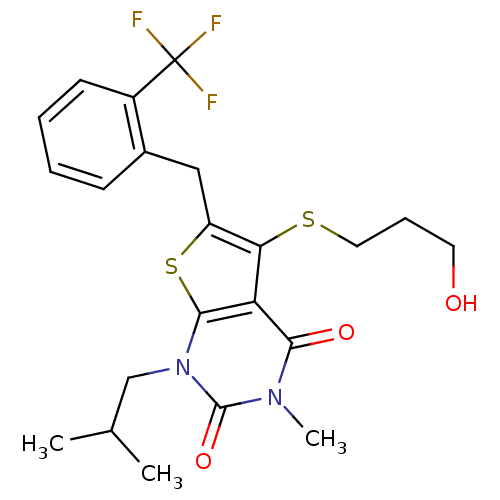
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266649
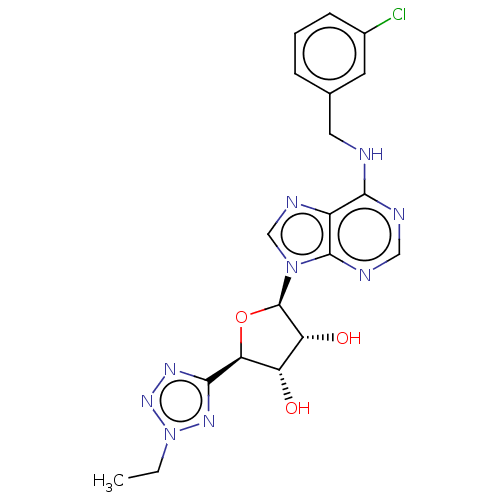
(CHEMBL4071338)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)ncnc12 |r| Show InChI InChI=1S/C19H20ClN9O3/c1-2-29-26-17(25-27-29)15-13(30)14(31)19(32-15)28-9-24-12-16(22-8-23-18(12)28)21-7-10-4-3-5-11(20)6-10/h3-6,8-9,13-15,19,30-31H,2,7H2,1H3,(H,21,22,23)/t13-,14+,15-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266650
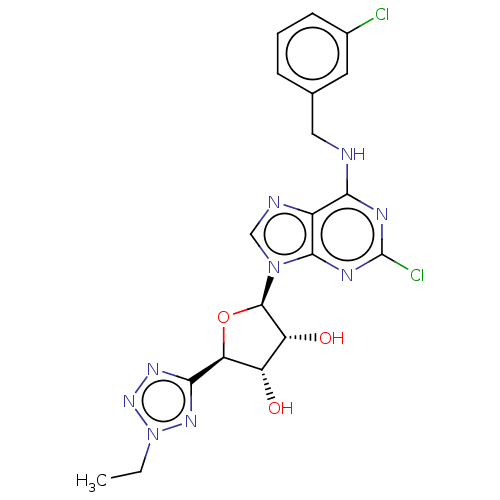
(CHEMBL4098377)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H19Cl2N9O3/c1-2-30-27-16(26-28-30)14-12(31)13(32)18(33-14)29-8-23-11-15(24-19(21)25-17(11)29)22-7-9-4-3-5-10(20)6-9/h3-6,8,12-14,18,31-32H,2,7H2,1H3,(H,22,24,25)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266651
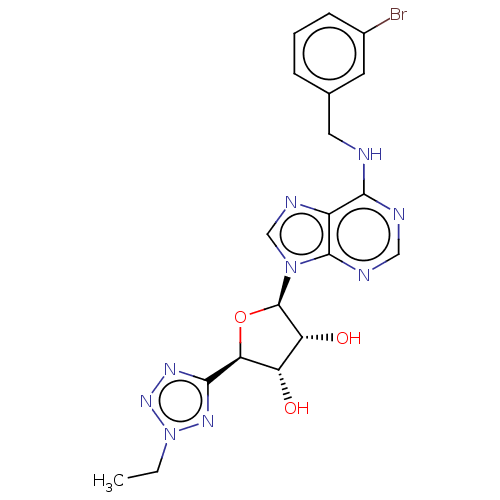
(CHEMBL4081876)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Br)c3)ncnc12 |r| Show InChI InChI=1S/C19H20BrN9O3/c1-2-29-26-17(25-27-29)15-13(30)14(31)19(32-15)28-9-24-12-16(22-8-23-18(12)28)21-7-10-4-3-5-11(20)6-10/h3-6,8-9,13-15,19,30-31H,2,7H2,1H3,(H,21,22,23)/t13-,14+,15-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50058163
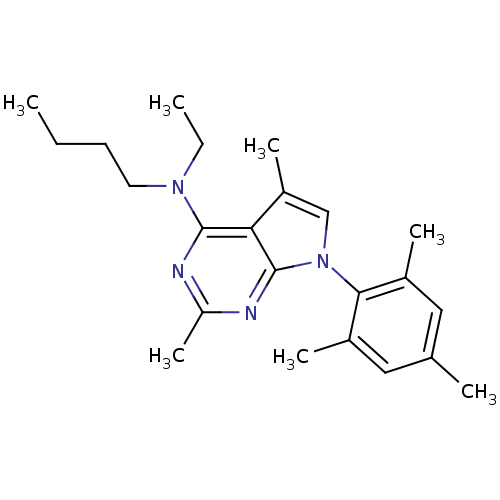
(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 57-69 (2003)
Article DOI: 10.1124/jpet.102.046128
BindingDB Entry DOI: 10.7270/Q20K273G |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599953

(CHEMBL1451229)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Br)ccc3-n12 |t:6| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247
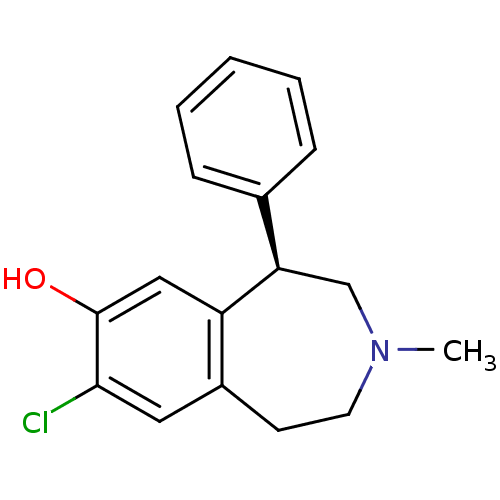
(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine receptor D1 using [3H]-SCH-23,390 was determined in rat striatal membranes |
J Med Chem 37: 4317-28 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2NC607Q |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50087713
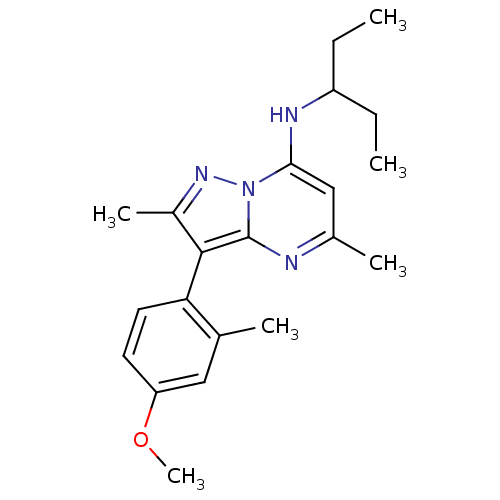
((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...)Show SMILES CCC(CC)Nc1cc(C)nc2c(c(C)nn12)-c1ccc(OC)cc1C |(-4.03,1.25,;-2.68,.48,;-1.35,1.27,;-1.37,2.81,;-2.86,3.21,;-.02,.5,;-.02,-1.04,;-1.34,-1.81,;-1.34,-3.35,;-2.66,-4.12,;,-4.12,;1.33,-3.35,;2.8,-3.8,;3.7,-2.55,;5.24,-2.53,;2.77,-1.32,;1.33,-1.81,;3.3,-5.26,;2.28,-6.39,;2.77,-7.87,;4.28,-8.16,;4.78,-9.63,;3.76,-10.79,;5.3,-7,;4.79,-5.55,;5.82,-4.4,)| Show InChI InChI=1S/C21H28N4O/c1-7-16(8-2)23-19-12-14(4)22-21-20(15(5)24-25(19)21)18-10-9-17(26-6)11-13(18)3/h9-12,16,23H,7-8H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 57-69 (2003)
Article DOI: 10.1124/jpet.102.046128
BindingDB Entry DOI: 10.7270/Q20K273G |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002402
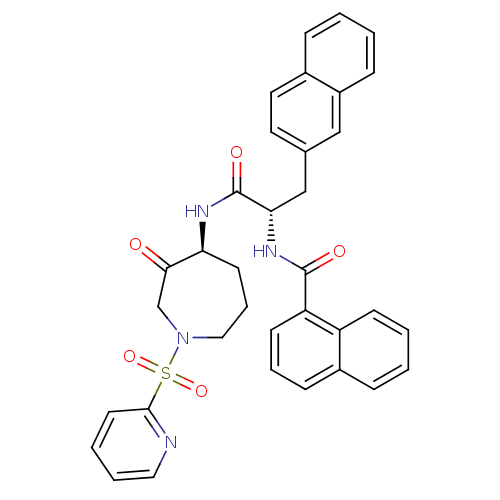
(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168
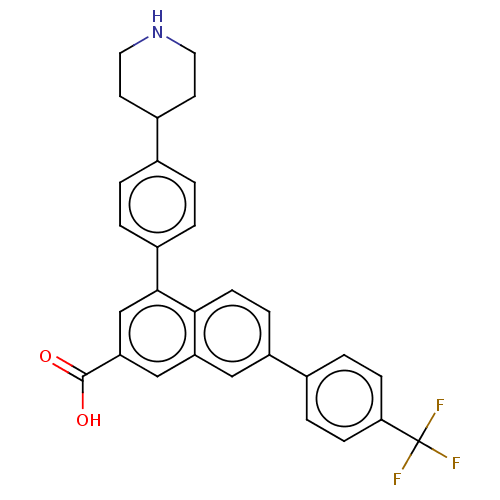
(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2Y14 expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP accumulation incubated for 15 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50266662
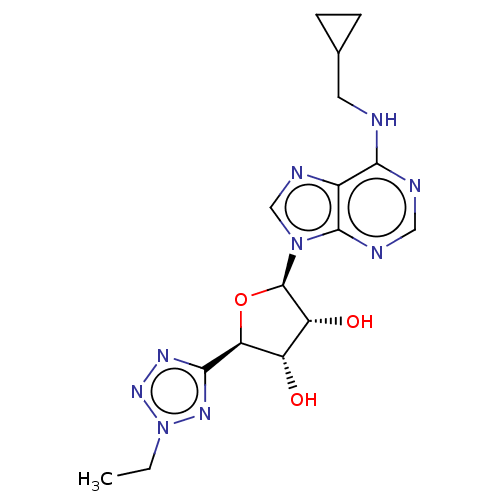
(CHEMBL4087306)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC3CC3)ncnc12 |r| Show InChI InChI=1S/C16H21N9O3/c1-2-25-22-14(21-23-25)12-10(26)11(27)16(28-12)24-7-20-9-13(17-5-8-3-4-8)18-6-19-15(9)24/h6-8,10-12,16,26-27H,2-5H2,1H3,(H,17,18,19)/t10-,11+,12-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM434802

(US10577368, Compound 100)Show SMILES CNC(=O)[C@]12CC1[C@H]([C@H](O)C2O)n1cnc2c(NC)nc(nc12)C#Cc1ccc(Br)s1 |r| Show InChI InChI=1S/C20H19BrN6O3S/c1-22-17-13-18(26-12(25-17)6-4-9-3-5-11(21)31-9)27(8-24-13)14-10-7-20(10,19(30)23-2)16(29)15(14)28/h3,5,8,10,14-16,28-29H,7H2,1-2H3,(H,23,30)(H,22,25,26)/t10?,14-,15+,16?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University
US Patent
| Assay Description
[3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... |
US Patent US10577368 (2020)
BindingDB Entry DOI: 10.7270/Q2HH6NGV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50069813
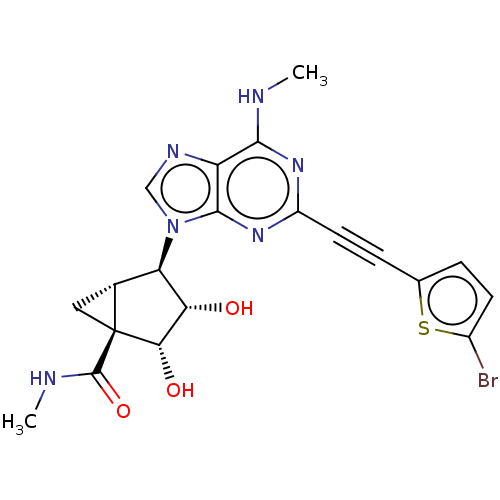
(CHEMBL3407785)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC)nc(nc12)C#Cc1ccc(Br)s1)C(=O)NC |r| Show InChI InChI=1S/C20H19BrN6O3S/c1-22-17-13-18(26-12(25-17)6-4-9-3-5-11(21)31-9)27(8-24-13)14-10-7-20(10,19(30)23-2)16(29)15(14)28/h3,5,8,10,14-16,28-29H,7H2,1-2H3,(H,23,30)(H,22,25,26)/t10-,14-,15+,16+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
J Med Chem 57: 9901-14 (2014)
Article DOI: 10.1021/jm501021n
BindingDB Entry DOI: 10.7270/Q2S1846G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50266666

(CHEMBL4105164)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC3CC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H20ClN9O3/c1-2-26-23-13(22-24-26)11-9(27)10(28)15(29-11)25-6-19-8-12(18-5-7-3-4-7)20-16(17)21-14(8)25/h6-7,9-11,15,27-28H,2-5H2,1H3,(H,18,20,21)/t9-,10+,11-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50189920

((S)-2-amino-N-((S)-2-(2-(benzylamino)-2-oxoethyl)-...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1Cc2ccccc2CN(CC(=O)NCc2ccccc2)C1=O |r| Show InChI InChI=1S/C30H34N4O4/c1-19-12-24(35)13-20(2)25(19)15-26(31)29(37)33-27-14-22-10-6-7-11-23(22)17-34(30(27)38)18-28(36)32-16-21-8-4-3-5-9-21/h3-13,26-27,35H,14-18,31H2,1-2H3,(H,32,36)(H,33,37)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes |
Bioorg Med Chem Lett 19: 433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.051
BindingDB Entry DOI: 10.7270/Q2K64HZ7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50078427
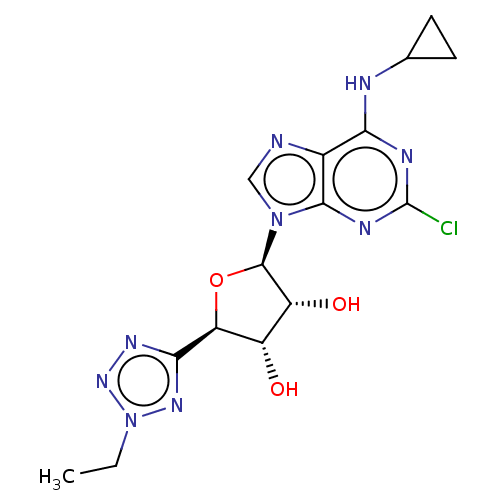
(CHEMBL3414941)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H18ClN9O3/c1-2-25-22-12(21-23-25)10-8(26)9(27)14(28-10)24-5-17-7-11(18-6-3-4-6)19-15(16)20-13(7)24/h5-6,8-10,14,26-27H,2-4H2,1H3,(H,18,19,20)/t8-,9+,10-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.461 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084650

(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599953

(CHEMBL1451229)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Br)ccc3-n12 |t:6| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM434800
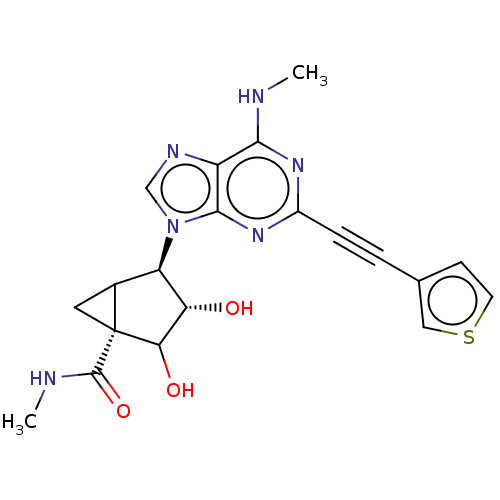
(US10577368, Compound 16)Show SMILES CNC(=O)[C@]12CC1[C@H]([C@H](O)C2O)n1cnc2c(NC)nc(nc12)C#Cc1ccsc1 |r| Show InChI InChI=1S/C20H20N6O3S/c1-21-17-13-18(25-12(24-17)4-3-10-5-6-30-8-10)26(9-23-13)14-11-7-20(11,19(29)22-2)16(28)15(14)27/h5-6,8-9,11,14-16,27-28H,7H2,1-2H3,(H,22,29)(H,21,24,25)/t11?,14-,15+,16?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University
US Patent
| Assay Description
[3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... |
US Patent US10577368 (2020)
BindingDB Entry DOI: 10.7270/Q2HH6NGV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data