 Found 6338 hits with Last Name = 'ray' and Initial = 'd'
Found 6338 hits with Last Name = 'ray' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486975
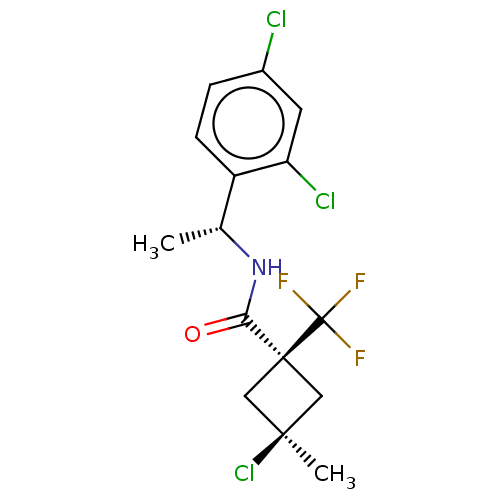
(CHEMBL2251221)Show SMILES C[C@@H](NC(=O)[C@@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Cl)cc1Cl |r,wU:7.8,wD:5.4,1.0,(9.6,-20.54,;9.61,-22.08,;8.29,-22.87,;6.95,-22.11,;6.93,-20.57,;5.62,-22.9,;4.51,-23.96,;3.44,-22.85,;2.34,-23.93,;2.1,-22.07,;4.55,-21.79,;6.7,-23.98,;6.31,-25.47,;8.19,-23.58,;7.79,-25.07,;10.95,-22.84,;10.96,-24.38,;12.3,-25.13,;13.63,-24.35,;14.97,-25.1,;13.61,-22.8,;12.27,-22.05,;12.24,-20.51,)| Show InChI InChI=1S/C15H15Cl3F3NO/c1-8(10-4-3-9(16)5-11(10)17)22-12(23)14(15(19,20)21)6-13(2,18)7-14/h3-5,8H,6-7H2,1-2H3,(H,22,23)/t8-,13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486958
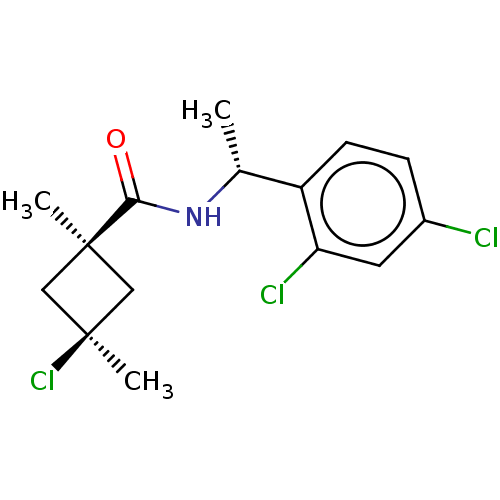
(CHEMBL2251869)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1)c1ccc(Cl)cc1Cl |r,wU:5.4,8.9,wD:1.0,(10.61,-1.98,;10.62,-3.52,;9.3,-4.3,;7.96,-3.55,;7.94,-2.01,;6.63,-4.33,;6.62,-5.86,;5.55,-5.43,;4.45,-4.34,;3.11,-5.1,;3.35,-3.24,;5.54,-3.25,;11.97,-4.27,;11.97,-5.81,;13.31,-6.57,;14.64,-5.78,;15.98,-6.54,;14.62,-4.24,;13.28,-3.49,;13.25,-1.95,)| Show InChI InChI=1S/C15H18Cl3NO/c1-9(11-5-4-10(16)6-12(11)17)19-13(20)14(2)7-15(3,18)8-14/h4-6,9H,7-8H2,1-3H3,(H,19,20)/t9-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486961
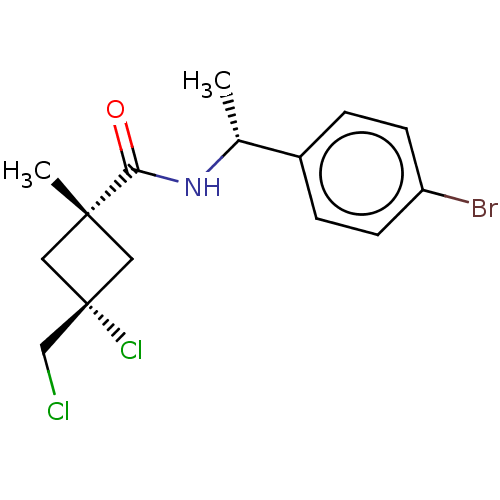
(CHEMBL2251863)Show SMILES C[C@@H](NC(=O)[C@]1(C)C[C@@](Cl)(CCl)C1)c1ccc(Br)cc1 |r,wU:8.9,wD:5.4,1.0,(9.94,-41.23,;9.96,-42.77,;8.63,-43.55,;7.29,-42.8,;7.27,-41.26,;5.97,-43.58,;5.95,-45.11,;4.88,-44.67,;3.78,-43.59,;2.68,-42.49,;2.44,-44.35,;1.11,-43.57,;4.87,-42.5,;11.3,-43.52,;11.31,-45.06,;12.65,-45.82,;13.97,-45.03,;15.32,-45.79,;13.95,-43.48,;12.61,-42.74,)| Show InChI InChI=1S/C15H18BrCl2NO/c1-10(11-3-5-12(16)6-4-11)19-13(20)14(2)7-15(18,8-14)9-17/h3-6,10H,7-9H2,1-2H3,(H,19,20)/t10-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486969
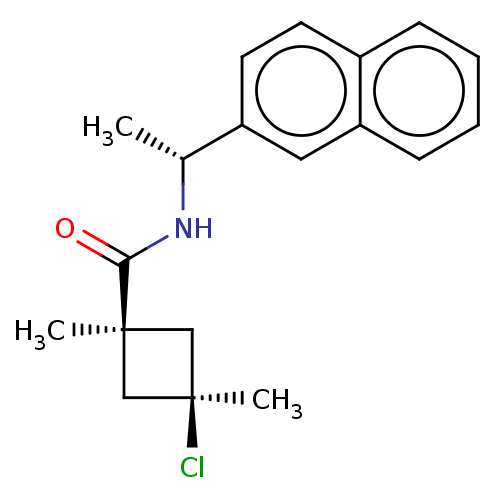
(CHEMBL2251871)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1)c1ccc2ccccc2c1 |r,wU:5.4,8.9,wD:1.0,(40.37,-2.17,;40.39,-3.71,;39.06,-4.5,;37.72,-3.74,;37.71,-2.2,;36.4,-4.52,;36.39,-6.06,;35.31,-5.62,;34.22,-4.54,;32.88,-5.3,;33.12,-3.44,;35.3,-3.44,;41.73,-4.47,;41.74,-6.01,;43.08,-6.76,;44.41,-5.98,;45.75,-6.73,;47.08,-5.93,;47.05,-4.38,;45.7,-3.64,;44.38,-4.43,;43.04,-3.68,)| Show InChI InChI=1S/C19H22ClNO/c1-13(21-17(22)18(2)11-19(3,20)12-18)15-9-8-14-6-4-5-7-16(14)10-15/h4-10,13H,11-12H2,1-3H3,(H,21,22)/t13-,18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486960

(CHEMBL2251864)Show SMILES CC[C@@]1(C[C@@](C)(Cl)C1)C(=O)N[C@H](C)c1ccc(Br)cc1 |r,wU:2.8,4.5,wD:11.12,(22.61,-45.48,;21.28,-44.7,;21.3,-43.16,;20.21,-44.26,;19.11,-43.18,;17.77,-43.94,;18.01,-42.08,;20.2,-42.08,;22.62,-42.38,;22.6,-40.84,;23.96,-43.14,;25.29,-42.36,;25.27,-40.82,;26.63,-43.11,;26.64,-44.65,;27.98,-45.4,;29.3,-44.62,;30.65,-45.37,;29.28,-43.07,;27.94,-42.32,)| Show InChI InChI=1S/C16H21BrClNO/c1-4-16(9-15(3,18)10-16)14(20)19-11(2)12-5-7-13(17)8-6-12/h5-8,11H,4,9-10H2,1-3H3,(H,19,20)/t11-,15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486966

(CHEMBL2114191)Show SMILES C[C@@H](NC(=O)[C@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Br)cc1 |wU:5.4,wD:7.7,1.0,(6.11,-1.45,;6.11,-2.99,;4.79,-3.76,;3.47,-2.99,;3.47,-1.45,;1.92,-2.99,;.83,-4.07,;-.25,-2.98,;-1.03,-1.65,;-1.34,-4.05,;.84,-1.9,;2.32,-4.47,;2.71,-5.97,;1.28,-5.9,;3.92,-5.2,;7.45,-3.76,;7.45,-5.3,;8.79,-6.07,;10.12,-5.3,;11.45,-6.07,;10.12,-3.76,;8.79,-2.99,)| Show InChI InChI=1S/C15H16BrClF3NO/c1-9(10-3-5-11(16)6-4-10)21-12(22)14(15(18,19)20)7-13(2,17)8-14/h3-6,9H,7-8H2,1-2H3,(H,21,22)/t9-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486974
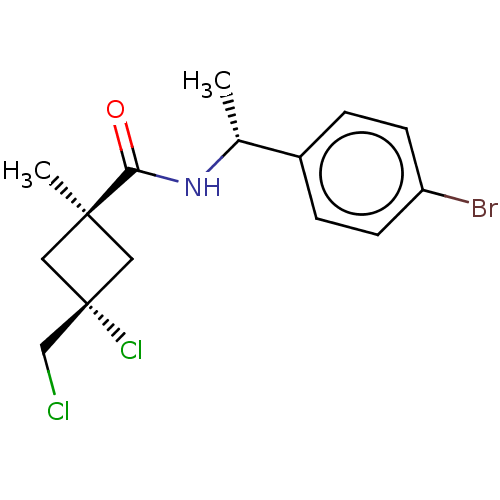
(CHEMBL2251862)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](Cl)(CCl)C1)c1ccc(Br)cc1 |r,wU:5.4,8.9,wD:1.0,(40.03,-33.88,;40.05,-35.42,;38.72,-36.2,;37.38,-35.45,;37.37,-33.91,;36.06,-36.23,;36.04,-37.76,;34.97,-37.32,;33.88,-36.24,;32.54,-37,;32.78,-35.14,;33.17,-33.65,;34.96,-35.15,;41.39,-36.17,;41.4,-37.71,;42.74,-38.47,;44.07,-37.68,;45.41,-38.44,;44.05,-36.13,;42.7,-35.39,)| Show InChI InChI=1S/C15H18BrCl2NO/c1-10(11-3-5-12(16)6-4-11)19-13(20)14(2)7-15(18,8-14)9-17/h3-6,10H,7-9H2,1-2H3,(H,19,20)/t10-,14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486972

(CHEMBL2251872)Show SMILES COc1cc(Br)ccc1C(C)NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1 |r,wU:14.14,17.19,(13.59,-9.79,;12.27,-10.58,;12.29,-12.12,;13.63,-12.87,;13.65,-14.42,;15,-15.17,;12.33,-15.2,;10.99,-14.45,;10.98,-12.91,;9.64,-12.15,;9.62,-10.61,;8.31,-12.94,;6.97,-12.18,;6.95,-10.64,;5.65,-12.96,;5.63,-14.5,;4.56,-14.06,;3.46,-12.97,;2.12,-13.74,;2.36,-11.88,;4.55,-11.88,)| Show InChI InChI=1S/C16H21BrClNO2/c1-10(12-6-5-11(17)7-13(12)21-4)19-14(20)15(2)8-16(3,18)9-15/h5-7,10H,8-9H2,1-4H3,(H,19,20)/t10?,15-,16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486967
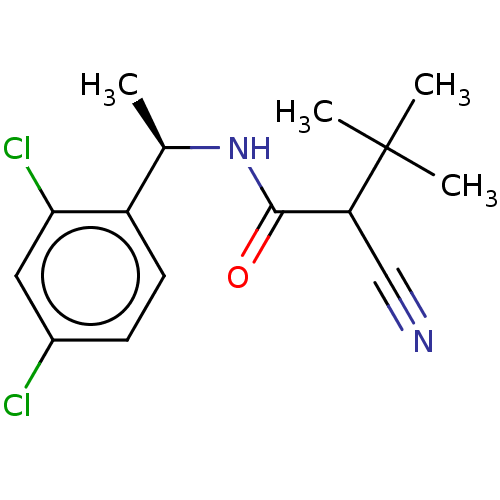
(CHEBI:81799 | DICLOCYMET)Show SMILES C[C@@H](NC(=O)C(C#N)C(C)(C)C)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C15H18Cl2N2O/c1-9(11-6-5-10(16)7-13(11)17)19-14(20)12(8-18)15(2,3)4/h5-7,9,12H,1-4H3,(H,19,20)/t9-,12?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433530
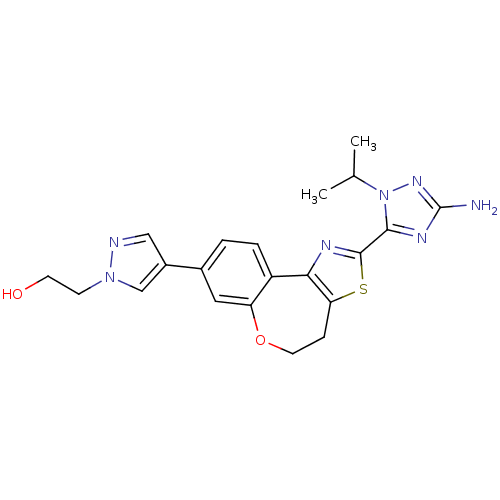
(CHEMBL2381382)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H23N7O2S/c1-12(2)28-19(25-21(22)26-28)20-24-18-15-4-3-13(14-10-23-27(11-14)6-7-29)9-16(15)30-8-5-17(18)31-20/h3-4,9-12,29H,5-8H2,1-2H3,(H2,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50078312
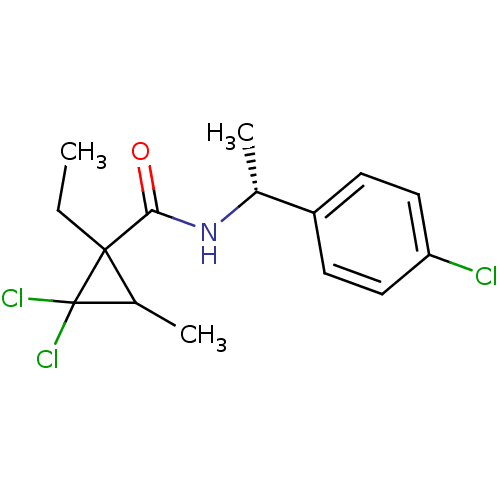
(2,2-Dichloro-1-ethyl-3-methyl-cyclopropanecarboxyl...)Show SMILES CCC1(C(C)C1(Cl)Cl)C(=O)N[C@H](C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H18Cl3NO/c1-4-14(10(3)15(14,17)18)13(20)19-9(2)11-5-7-12(16)8-6-11/h5-10H,4H2,1-3H3,(H,19,20)/t9-,10?,14?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486952
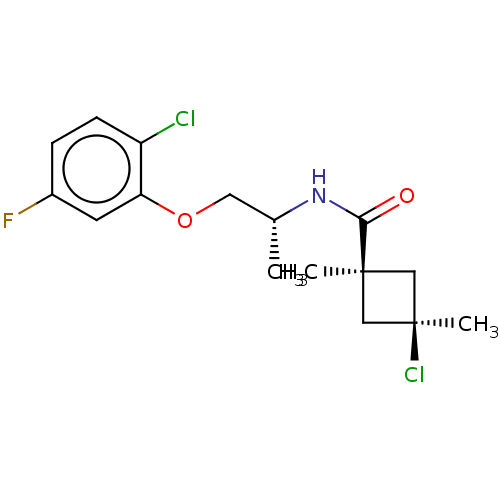
(CHEMBL2251854)Show SMILES C[C@H](COc1cc(F)ccc1Cl)NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1 |r,wU:15.15,18.20,wD:1.0,(41.6,-11.34,;41.61,-12.88,;42.95,-13.63,;44.28,-12.85,;45.62,-13.6,;45.63,-15.14,;46.97,-15.89,;46.99,-17.43,;48.3,-15.11,;48.28,-13.56,;46.94,-12.81,;46.91,-11.27,;40.29,-13.66,;38.95,-12.9,;38.93,-11.36,;37.62,-13.68,;37.61,-15.22,;36.53,-14.78,;35.44,-13.7,;34.1,-14.46,;34.34,-12.6,;36.53,-12.6,)| Show InChI InChI=1S/C16H20Cl2FNO2/c1-10(7-22-13-6-11(19)4-5-12(13)17)20-14(21)15(2)8-16(3,18)9-15/h4-6,10H,7-9H2,1-3H3,(H,20,21)/t10-,15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433534
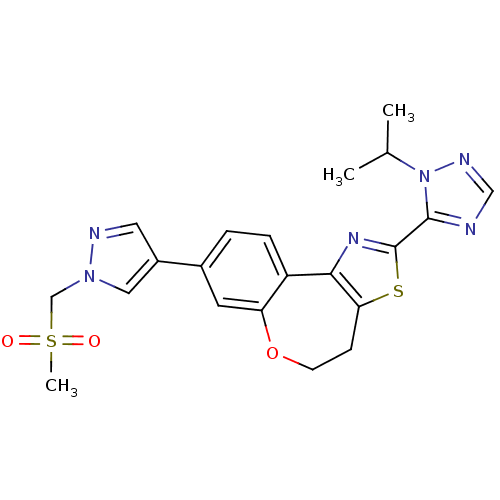
(CHEMBL2381375)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CS(C)(=O)=O)c2)s1 Show InChI InChI=1S/C21H22N6O3S2/c1-13(2)27-20(22-11-24-27)21-25-19-16-5-4-14(8-17(16)30-7-6-18(19)31-21)15-9-23-26(10-15)12-32(3,28)29/h4-5,8-11,13H,6-7,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433533
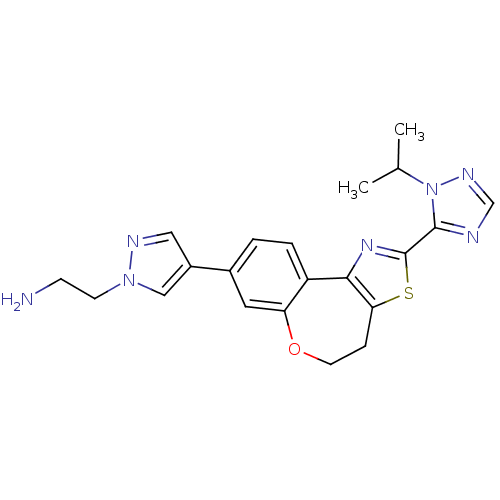
(CHEMBL2381376)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCN)c2)s1 Show InChI InChI=1S/C21H23N7OS/c1-13(2)28-20(23-12-25-28)21-26-19-16-4-3-14(15-10-24-27(11-15)7-6-22)9-17(16)29-8-5-18(19)30-21/h3-4,9-13H,5-8,22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486954
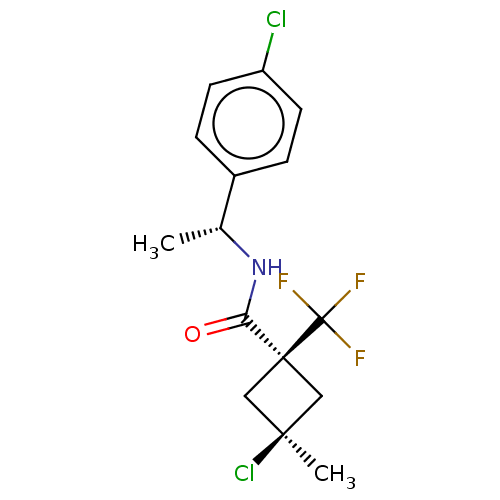
(CHEMBL2251875)Show SMILES C[C@@H](NC(=O)[C@@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Cl)cc1 |r,wU:7.8,wD:1.0,5.4,(29.18,-22.69,;29.2,-24.23,;27.87,-25.01,;26.53,-24.26,;26.51,-22.72,;25.2,-25.04,;24.09,-26.11,;23.02,-25,;21.92,-26.08,;21.68,-24.22,;24.14,-23.93,;25.97,-26.37,;25.21,-27.71,;26.74,-27.7,;27.44,-26.76,;30.54,-24.99,;31.85,-24.2,;33.19,-24.95,;33.21,-26.5,;34.56,-27.25,;31.89,-27.28,;30.55,-26.52,)| Show InChI InChI=1S/C15H16Cl2F3NO/c1-9(10-3-5-11(16)6-4-10)21-12(22)14(15(18,19)20)7-13(2,17)8-14/h3-6,9H,7-8H2,1-2H3,(H,21,22)/t9-,13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50427363
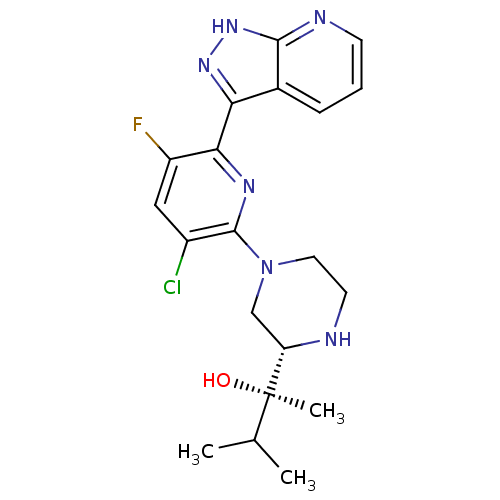
(CHEMBL2326002)Show SMILES CC(C)[C@@](C)(O)[C@@H]1CN(CCN1)c1nc(-c2n[nH]c3ncccc23)c(F)cc1Cl |r| Show InChI InChI=1S/C20H24ClFN6O/c1-11(2)20(3,29)15-10-28(8-7-23-15)19-13(21)9-14(22)17(25-19)16-12-5-4-6-24-18(12)27-26-16/h4-6,9,11,15,23,29H,7-8,10H2,1-3H3,(H,24,26,27)/t15-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... |
J Med Chem 56: 1799-810 (2013)
Article DOI: 10.1021/jm301465a
BindingDB Entry DOI: 10.7270/Q2M046R2 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486953

(CHEMBL2251861)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1)c1ccc(Br)cc1 |r,wU:5.4,8.9,wD:1.0,(24.86,-34.05,;24.87,-35.59,;23.55,-36.37,;22.21,-35.62,;22.19,-34.08,;20.88,-36.4,;20.87,-37.93,;19.8,-37.5,;18.7,-36.41,;17.36,-37.17,;17.6,-35.31,;19.79,-35.32,;26.22,-36.34,;26.22,-37.88,;27.56,-38.64,;28.89,-37.85,;30.23,-38.61,;28.87,-36.31,;27.53,-35.56,)| Show InChI InChI=1S/C15H19BrClNO/c1-10(11-4-6-12(16)7-5-11)18-13(19)14(2)8-15(3,17)9-14/h4-7,10H,8-9H2,1-3H3,(H,18,19)/t10-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50427365
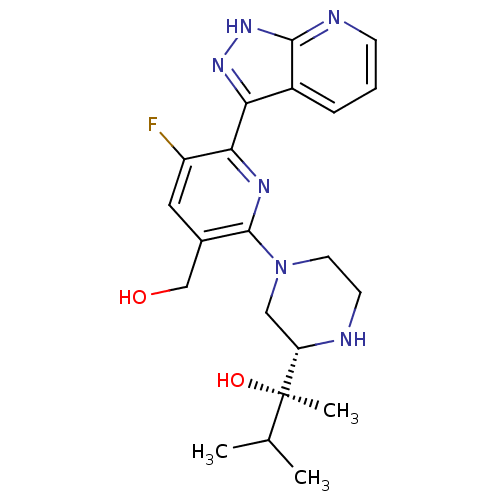
(CHEMBL2326000)Show SMILES CC(C)[C@@](C)(O)[C@@H]1CN(CCN1)c1nc(-c2n[nH]c3ncccc23)c(F)cc1CO |r| Show InChI InChI=1S/C21H27FN6O2/c1-12(2)21(3,30)16-10-28(8-7-23-16)20-13(11-29)9-15(22)18(25-20)17-14-5-4-6-24-19(14)27-26-17/h4-6,9,12,16,23,29-30H,7-8,10-11H2,1-3H3,(H,24,26,27)/t16-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... |
J Med Chem 56: 1799-810 (2013)
Article DOI: 10.1021/jm301465a
BindingDB Entry DOI: 10.7270/Q2M046R2 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50427364
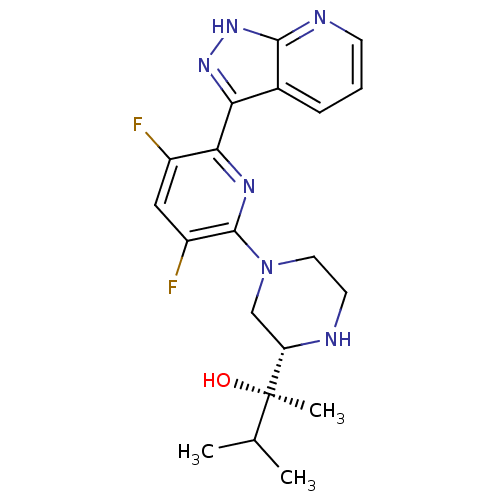
(CHEMBL2326001)Show SMILES CC(C)[C@@](C)(O)[C@@H]1CN(CCN1)c1nc(-c2n[nH]c3ncccc23)c(F)cc1F |r| Show InChI InChI=1S/C20H24F2N6O/c1-11(2)20(3,29)15-10-28(8-7-23-15)19-14(22)9-13(21)17(25-19)16-12-5-4-6-24-18(12)27-26-16/h4-6,9,11,15,23,29H,7-8,10H2,1-3H3,(H,24,26,27)/t15-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... |
J Med Chem 56: 1799-810 (2013)
Article DOI: 10.1021/jm301465a
BindingDB Entry DOI: 10.7270/Q2M046R2 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50427367
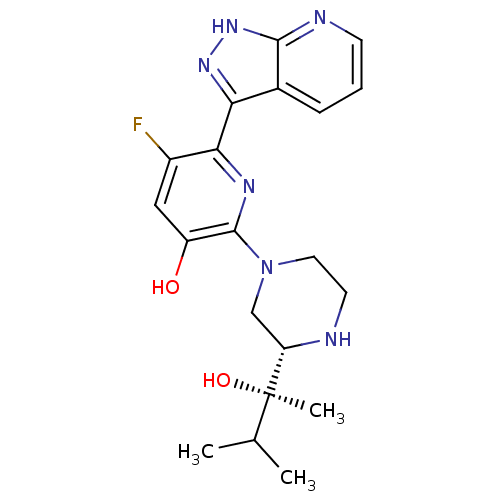
(CHEMBL2325998)Show SMILES CC(C)[C@@](C)(O)[C@@H]1CN(CCN1)c1nc(-c2n[nH]c3ncccc23)c(F)cc1O |r| Show InChI InChI=1S/C20H25FN6O2/c1-11(2)20(3,29)15-10-27(8-7-22-15)19-14(28)9-13(21)17(24-19)16-12-5-4-6-23-18(12)26-25-16/h4-6,9,11,15,22,28-29H,7-8,10H2,1-3H3,(H,23,25,26)/t15-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... |
J Med Chem 56: 1799-810 (2013)
Article DOI: 10.1021/jm301465a
BindingDB Entry DOI: 10.7270/Q2M046R2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433532
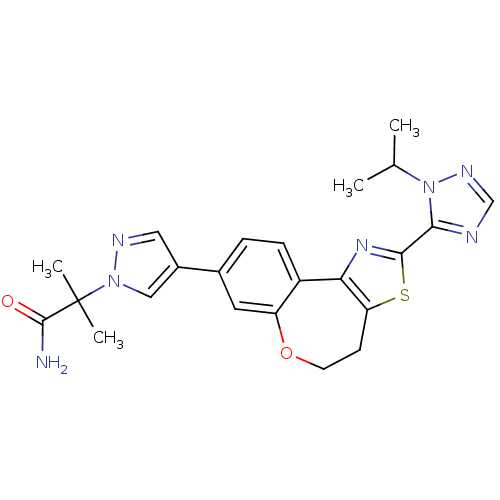
(CHEMBL2381377)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(c2)C(C)(C)C(N)=O)s1 Show InChI InChI=1S/C23H25N7O2S/c1-13(2)30-20(25-12-27-30)21-28-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-26-29(11-15)23(3,4)22(24)31/h5-6,9-13H,7-8H2,1-4H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486956
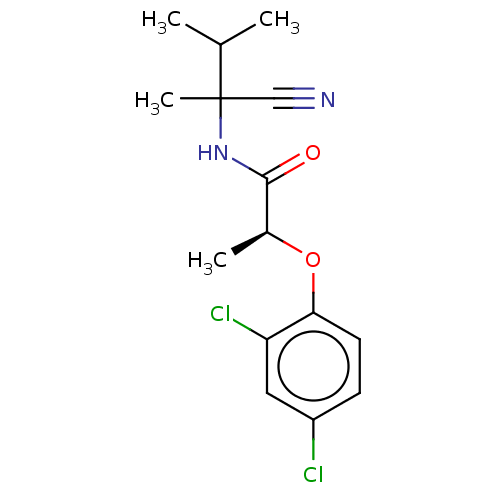
(CHEMBL2251991)Show SMILES CC(C)C(C)(NC(=O)[C@H](C)Oc1ccc(Cl)cc1Cl)C#N |r| Show InChI InChI=1S/C15H18Cl2N2O2/c1-9(2)15(4,8-18)19-14(20)10(3)21-13-6-5-11(16)7-12(13)17/h5-7,9-10H,1-4H3,(H,19,20)/t10-,15?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433529
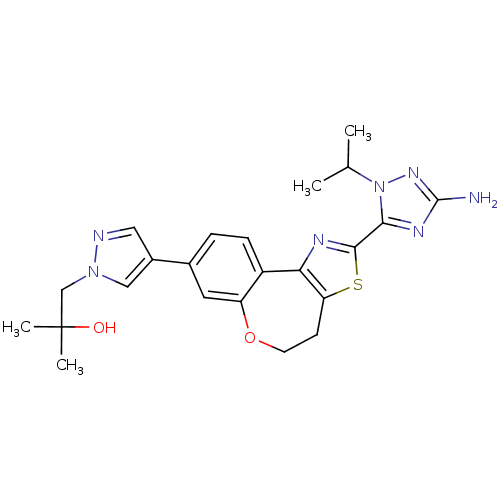
(CHEMBL2381380)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H27N7O2S/c1-13(2)30-20(27-22(24)28-30)21-26-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-25-29(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433527

(CHEMBL2381379)Show SMILES CC(C)(O)Cn1cc(cn1)-c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C22H21F3N6O2S/c1-21(2,32)10-30-9-14(8-27-30)13-3-4-15-16(7-13)33-6-5-17-18(15)29-20(34-17)19-26-12-28-31(19)11-22(23,24)25/h3-4,7-9,12,32H,5-6,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433535
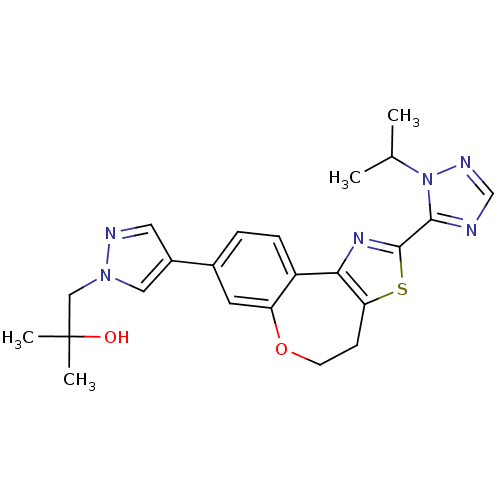
(CHEMBL2381374)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O2S/c1-14(2)29-21(24-13-26-29)22-27-20-17-6-5-15(9-18(17)31-8-7-19(20)32-22)16-10-25-28(11-16)12-23(3,4)30/h5-6,9-11,13-14,30H,7-8,12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
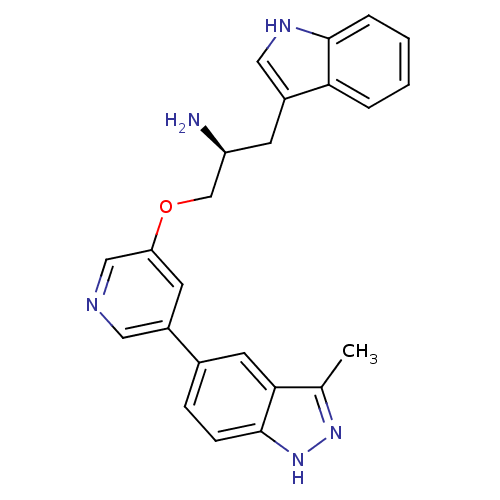
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Nat Chem Biol 5: 484-93 (2009)
Article DOI: 10.1038/nchembio.183
BindingDB Entry DOI: 10.7270/Q2D21XTB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
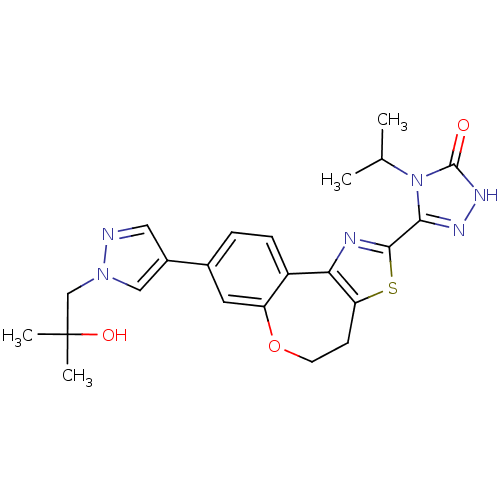
(Homo sapiens (Human)) | BDBM50433528
(CHEMBL2381381)Show SMILES CC(C)n1c(n[nH]c1=O)-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O3S/c1-13(2)29-20(26-27-22(29)30)21-25-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-24-28(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
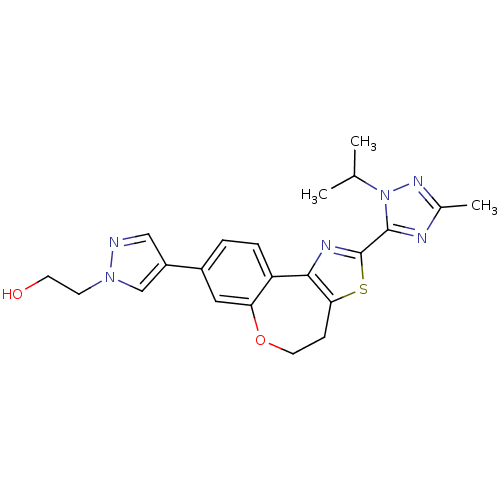
(Homo sapiens (Human)) | BDBM50433531
(CHEMBL2381378)Show SMILES CC(C)n1nc(C)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C22H24N6O2S/c1-13(2)28-21(24-14(3)26-28)22-25-20-17-5-4-15(16-11-23-27(12-16)7-8-29)10-18(17)30-9-6-19(20)31-22/h4-5,10-13,29H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
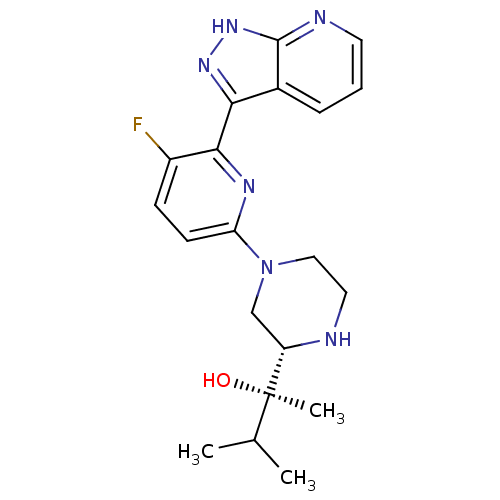
(Homo sapiens (Human)) | BDBM50427370
(CHEMBL2326007)Show SMILES CC(C)[C@@](C)(O)[C@@H]1CN(CCN1)c1ccc(F)c(n1)-c1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C20H25FN6O/c1-12(2)20(3,28)15-11-27(10-9-22-15)16-7-6-14(21)18(24-16)17-13-5-4-8-23-19(13)26-25-17/h4-8,12,15,22,28H,9-11H2,1-3H3,(H,23,25,26)/t15-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... |
J Med Chem 56: 1799-810 (2013)
Article DOI: 10.1021/jm301465a
BindingDB Entry DOI: 10.7270/Q2M046R2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B |
Bioorg Med Chem Lett 15: 3203-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.001
BindingDB Entry DOI: 10.7270/Q2J67KP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433536
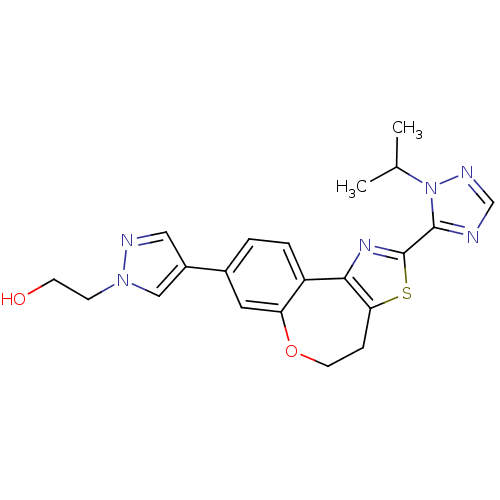
(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486962
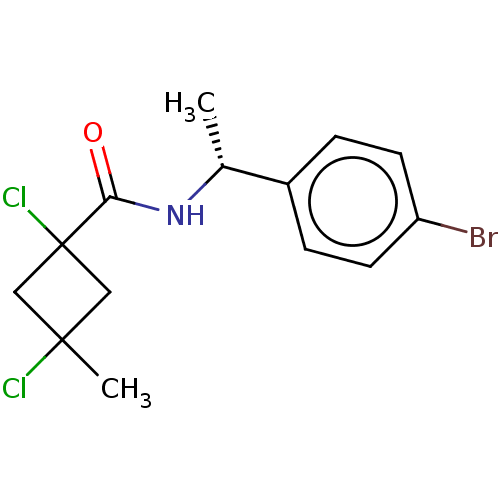
(CHEMBL2251860)Show SMILES C[C@@H](NC(=O)C1(Cl)CC(C)(Cl)C1)c1ccc(Br)cc1 |r,wD:1.0,(8.76,-34.34,;8.77,-35.88,;7.45,-36.66,;6.11,-35.9,;6.09,-34.36,;4.78,-36.68,;4.77,-38.22,;3.69,-37.78,;2.6,-36.7,;1.26,-37.46,;1.5,-35.6,;3.69,-35.6,;10.12,-36.63,;10.12,-38.17,;11.46,-38.93,;12.79,-38.14,;14.13,-38.9,;12.77,-36.59,;11.43,-35.84,)| Show InChI InChI=1S/C14H16BrCl2NO/c1-9(10-3-5-11(15)6-4-10)18-12(19)14(17)7-13(2,16)8-14/h3-6,9H,7-8H2,1-2H3,(H,18,19)/t9-,13?,14?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044676
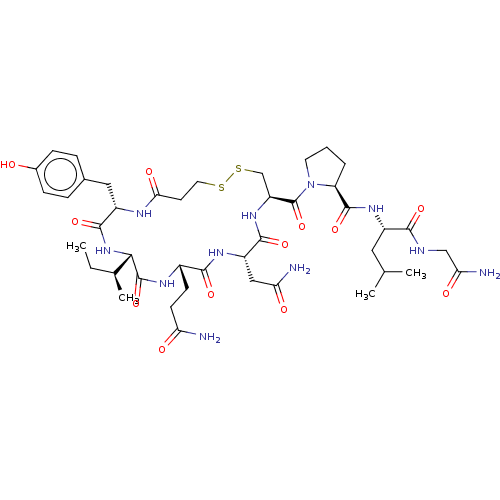
(CHEMBL439044)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C43H65N11O12S2/c1-5-23(4)36-42(65)49-26(12-13-32(44)56)38(61)50-29(19-33(45)57)39(62)52-30(21-68-67-16-14-35(59)48-28(40(63)53-36)18-24-8-10-25(55)11-9-24)43(66)54-15-6-7-31(54)41(64)51-27(17-22(2)3)37(60)47-20-34(46)58/h8-11,22-23,26-31,36,55H,5-7,12-21H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,60)(H,48,59)(H,49,65)(H,50,61)(H,51,64)(H,52,62)(H,53,63)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... |
Bioorg Med Chem 24: 3513-20 (2016)
Article DOI: 10.1016/j.bmc.2016.05.062
BindingDB Entry DOI: 10.7270/Q2WH2RXT |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371333
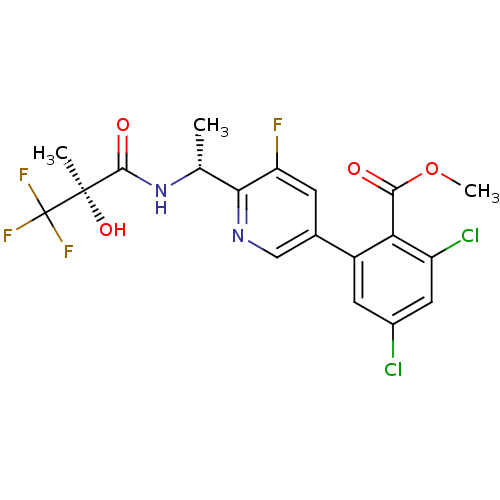
(CHEMBL256671)Show SMILES COC(=O)c1c(Cl)cc(Cl)cc1-c1cnc([C@@H](C)NC(=O)[C@@](C)(O)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C19H16Cl2F4N2O4/c1-8(27-17(29)18(2,30)19(23,24)25)15-13(22)4-9(7-26-15)11-5-10(20)6-12(21)14(11)16(28)31-3/h4-8,30H,1-3H3,(H,27,29)/t8-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradikinin B1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 716-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.050
BindingDB Entry DOI: 10.7270/Q2GM8849 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486964
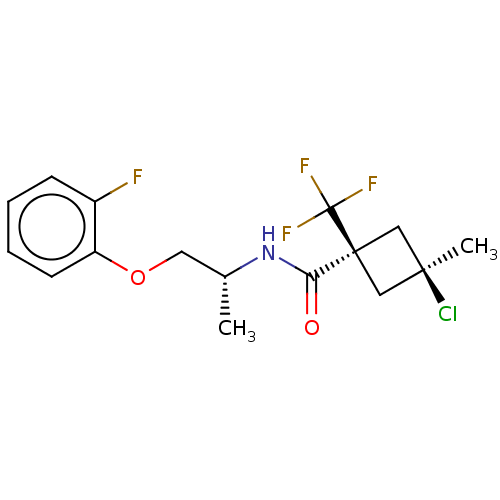
(CHEMBL2251855)Show SMILES C[C@H](COc1ccccc1F)NC(=O)[C@@]1(C[C@@](C)(Cl)C1)C(F)(F)F |r,wU:16.18,wD:14.14,1.0,(25.38,-23.54,;25.39,-25.08,;26.73,-25.83,;28.05,-25.06,;29.39,-25.81,;29.4,-27.35,;30.74,-28.11,;32.07,-27.32,;32.05,-25.77,;30.7,-25.03,;30.68,-23.48,;24.06,-25.86,;22.71,-25.11,;22.7,-23.56,;21.38,-25.89,;20.27,-26.95,;19.21,-25.84,;18.1,-26.92,;17.87,-25.07,;20.32,-24.78,;22.17,-27.21,;22.95,-28.54,;23.71,-27.19,;21.41,-28.55,)| Show InChI InChI=1S/C16H18ClF4NO2/c1-10(7-24-12-6-4-3-5-11(12)18)22-13(23)15(16(19,20)21)8-14(2,17)9-15/h3-6,10H,7-9H2,1-2H3,(H,22,23)/t10-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486968
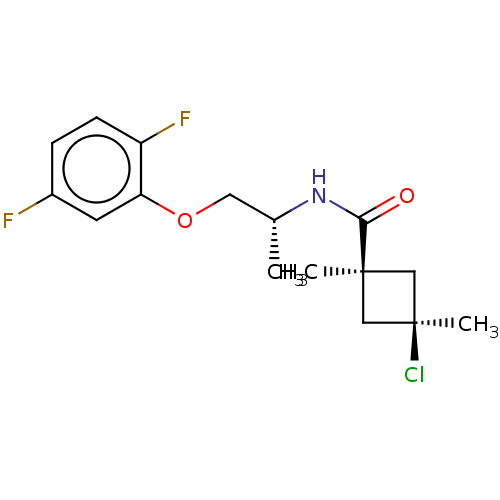
(CHEMBL2251873)Show SMILES C[C@H](COc1cc(F)ccc1F)NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1 |r,wU:15.15,18.20,wD:1.0,(25.12,-11.31,;25.14,-12.85,;26.48,-13.61,;27.81,-12.82,;29.15,-13.58,;29.16,-15.12,;30.5,-15.87,;30.51,-17.41,;31.82,-15.09,;31.8,-13.54,;30.46,-12.79,;30.44,-11.25,;23.81,-13.64,;22.47,-12.88,;22.45,-11.34,;21.15,-13.66,;21.13,-15.2,;20.06,-14.76,;18.97,-13.68,;17.63,-14.44,;17.87,-12.58,;20.05,-12.58,)| Show InChI InChI=1S/C16H20ClF2NO2/c1-10(7-22-13-6-11(18)4-5-12(13)19)20-14(21)15(2)8-16(3,17)9-15/h4-6,10H,7-9H2,1-3H3,(H,20,21)/t10-,15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371320
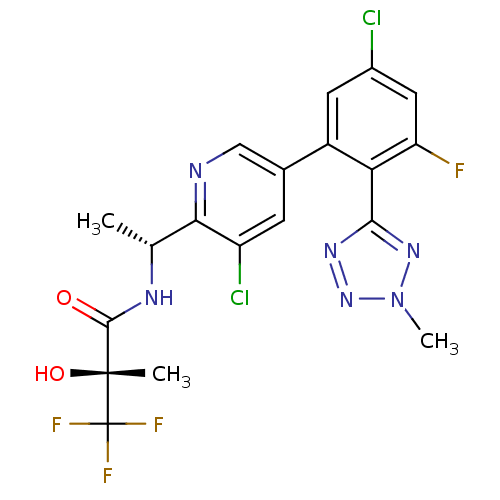
(CHEMBL271283)Show SMILES C[C@@H](NC(=O)[C@@](C)(O)C(F)(F)F)c1ncc(cc1Cl)-c1cc(Cl)cc(F)c1-c1nnn(C)n1 Show InChI InChI=1S/C19H16Cl2F4N6O2/c1-8(27-17(32)18(2,33)19(23,24)25)15-12(21)4-9(7-26-15)11-5-10(20)6-13(22)14(11)16-28-30-31(3)29-16/h4-8,33H,1-3H3,(H,27,32)/t8-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradikinin B1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 716-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.050
BindingDB Entry DOI: 10.7270/Q2GM8849 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486965
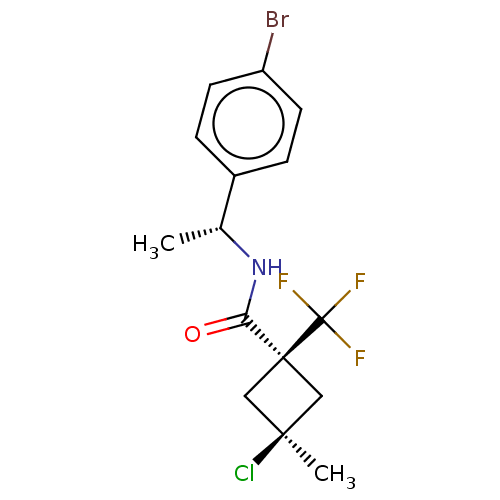
(CHEMBL2251874)Show SMILES C[C@@H](NC(=O)[C@@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Br)cc1 |r,wU:7.8,wD:5.4,1.0,(24.57,-11.74,;24.59,-13.28,;23.26,-14.06,;21.92,-13.31,;21.9,-11.77,;20.59,-14.09,;19.48,-15.16,;18.41,-14.05,;17.31,-15.13,;17.07,-13.27,;19.53,-12.98,;21.68,-15.17,;21.28,-16.66,;23.16,-14.77,;22.77,-16.26,;25.93,-14.04,;25.93,-15.57,;27.28,-16.33,;28.6,-15.54,;29.95,-16.3,;28.58,-14,;27.24,-13.25,)| Show InChI InChI=1S/C15H16BrClF3NO/c1-9(10-3-5-11(16)6-4-10)21-12(22)14(15(18,19)20)7-13(2,17)8-14/h3-6,9H,7-8H2,1-2H3,(H,21,22)/t9-,13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... |
Bioorg Med Chem 24: 3513-20 (2016)
Article DOI: 10.1016/j.bmc.2016.05.062
BindingDB Entry DOI: 10.7270/Q2WH2RXT |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486977

(CHEMBL2251858)Show SMILES C[C@@H](NC(=O)C1(Cl)CC(Cl)(Cl)C1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C13H13BrCl3NO/c1-8(9-2-4-10(14)5-3-9)18-11(19)12(15)6-13(16,17)7-12/h2-5,8H,6-7H2,1H3,(H,18,19)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433537
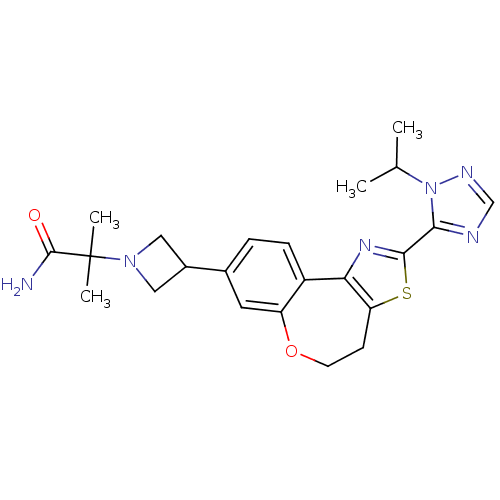
(CHEMBL2381279)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C2)C(C)(C)C(N)=O)s1 Show InChI InChI=1S/C23H28N6O2S/c1-13(2)29-20(25-12-26-29)21-27-19-16-6-5-14(9-17(16)31-8-7-18(19)32-21)15-10-28(11-15)23(3,4)22(24)30/h5-6,9,12-13,15H,7-8,10-11H2,1-4H3,(H2,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50433536

(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kgamma expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM160878
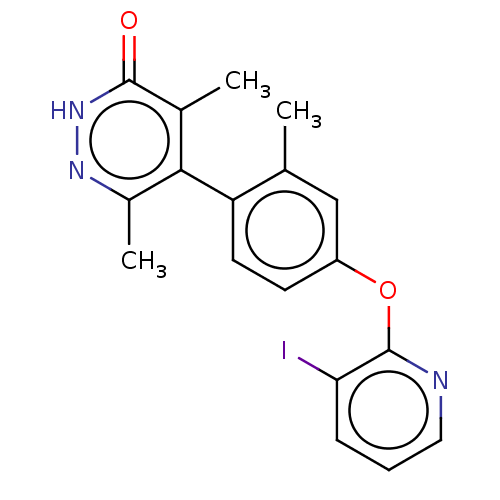
(US10093655, Example 48 | US11014909, Example 48 | ...)Show SMILES Cc1cc(Oc2ncccc2I)ccc1-c1c(C)n[nH]c(=O)c1C |(,3.47,;,1.93,;-1.33,1.15,;-1.33,-.38,;-2.67,-1.15,;-2.67,-2.69,;-1.33,-3.47,;-1.33,-5,;-2.67,-5.78,;-4,-5,;-4,-3.47,;-5.33,-2.69,;,-1.15,;1.33,-.38,;1.33,1.15,;2.67,1.93,;4,1.15,;4,-.38,;5.33,1.93,;5.33,3.47,;4,4.23,;4,5.78,;2.67,3.47,;1.33,4.23,)| Show InChI InChI=1S/C18H16IN3O2/c1-10-9-13(24-18-15(19)5-4-8-20-18)6-7-14(10)16-11(2)17(23)22-21-12(16)3/h4-9H,1-3H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... |
US Patent US9107923 (2015)
BindingDB Entry DOI: 10.7270/Q2C24V5T |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM160878

(US10093655, Example 48 | US11014909, Example 48 | ...)Show SMILES Cc1cc(Oc2ncccc2I)ccc1-c1c(C)n[nH]c(=O)c1C |(,3.47,;,1.93,;-1.33,1.15,;-1.33,-.38,;-2.67,-1.15,;-2.67,-2.69,;-1.33,-3.47,;-1.33,-5,;-2.67,-5.78,;-4,-5,;-4,-3.47,;-5.33,-2.69,;,-1.15,;1.33,-.38,;1.33,1.15,;2.67,1.93,;4,1.15,;4,-.38,;5.33,1.93,;5.33,3.47,;4,4.23,;4,5.78,;2.67,3.47,;1.33,4.23,)| Show InChI InChI=1S/C18H16IN3O2/c1-10-9-13(24-18-15(19)5-4-8-20-18)6-7-14(10)16-11(2)17(23)22-21-12(16)3/h4-9H,1-3H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... |
US Patent US11014909 (2021)
BindingDB Entry DOI: 10.7270/Q27D2Z7D |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM160878

(US10093655, Example 48 | US11014909, Example 48 | ...)Show SMILES Cc1cc(Oc2ncccc2I)ccc1-c1c(C)n[nH]c(=O)c1C |(,3.47,;,1.93,;-1.33,1.15,;-1.33,-.38,;-2.67,-1.15,;-2.67,-2.69,;-1.33,-3.47,;-1.33,-5,;-2.67,-5.78,;-4,-5,;-4,-3.47,;-5.33,-2.69,;,-1.15,;1.33,-.38,;1.33,1.15,;2.67,1.93,;4,1.15,;4,-.38,;5.33,1.93,;5.33,3.47,;4,4.23,;4,5.78,;2.67,3.47,;1.33,4.23,)| Show InChI InChI=1S/C18H16IN3O2/c1-10-9-13(24-18-15(19)5-4-8-20-18)6-7-14(10)16-11(2)17(23)22-21-12(16)3/h4-9H,1-3H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... |
US Patent US10093655 (2018)
BindingDB Entry DOI: 10.7270/Q2SQ92F2 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371332
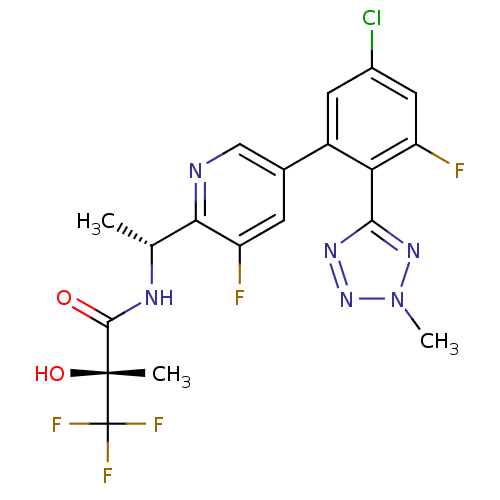
(CHEMBL258324)Show SMILES C[C@@H](NC(=O)[C@@](C)(O)C(F)(F)F)c1ncc(cc1F)-c1cc(Cl)cc(F)c1-c1nnn(C)n1 Show InChI InChI=1S/C19H16ClF5N6O2/c1-8(27-17(32)18(2,33)19(23,24)25)15-13(22)4-9(7-26-15)11-5-10(20)6-12(21)14(11)16-28-30-31(3)29-16/h4-8,33H,1-3H3,(H,27,32)/t8-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradikinin B1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 716-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.050
BindingDB Entry DOI: 10.7270/Q2GM8849 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-A by coupled assay |
Bioorg Med Chem Lett 19: 3586-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.136
BindingDB Entry DOI: 10.7270/Q28K7944 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50387328
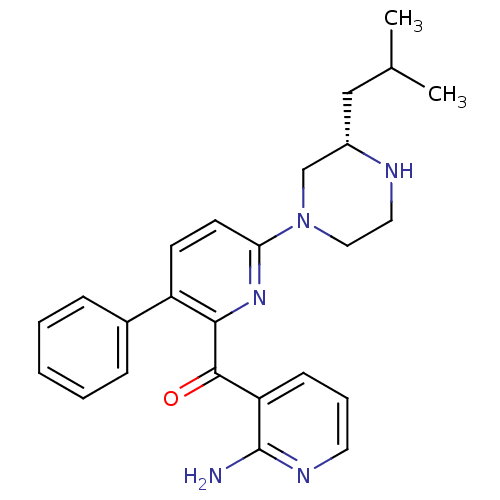
(CHEMBL2046649)Show SMILES CC(C)C[C@H]1CN(CCN1)c1ccc(-c2ccccc2)c(n1)C(=O)c1cccnc1N |r| Show InChI InChI=1S/C25H29N5O/c1-17(2)15-19-16-30(14-13-27-19)22-11-10-20(18-7-4-3-5-8-18)23(29-22)24(31)21-9-6-12-28-25(21)26/h3-12,17,19,27H,13-16H2,1-2H3,(H2,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry at Vertex Pharmaceuticals (Europe) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta |
Bioorg Med Chem Lett 22: 4645-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.114
BindingDB Entry DOI: 10.7270/Q2TM7C65 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50433536

(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kdelta expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433539
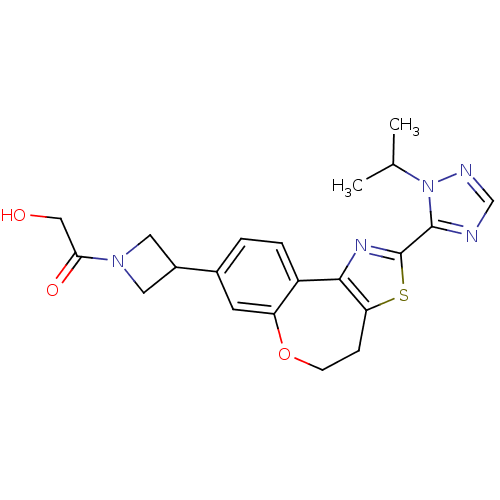
(CHEMBL2381277)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C2)C(=O)CO)s1 Show InChI InChI=1S/C21H23N5O3S/c1-12(2)26-20(22-11-23-26)21-24-19-15-4-3-13(14-8-25(9-14)18(28)10-27)7-16(15)29-6-5-17(19)30-21/h3-4,7,11-12,14,27H,5-6,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data