 Found 11612 hits with Last Name = 'smith' and Initial = 'd'
Found 11612 hits with Last Name = 'smith' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
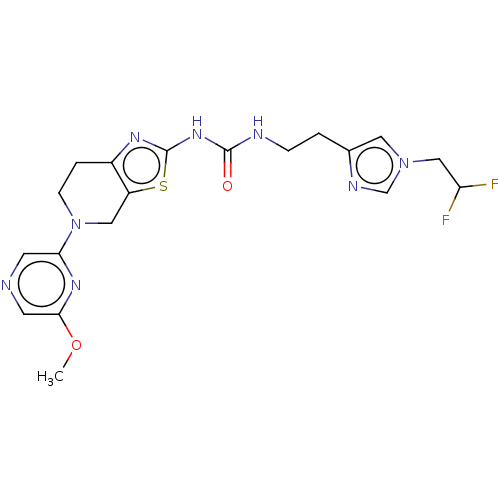
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
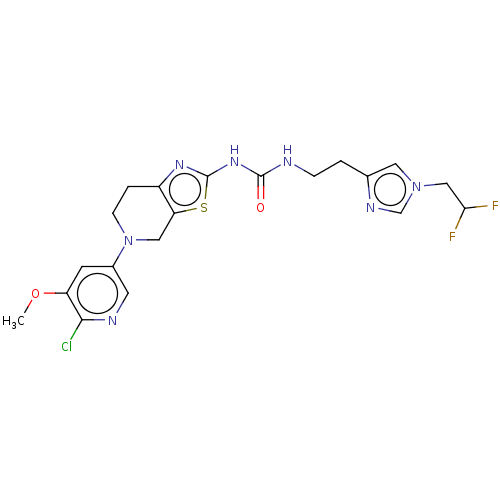
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
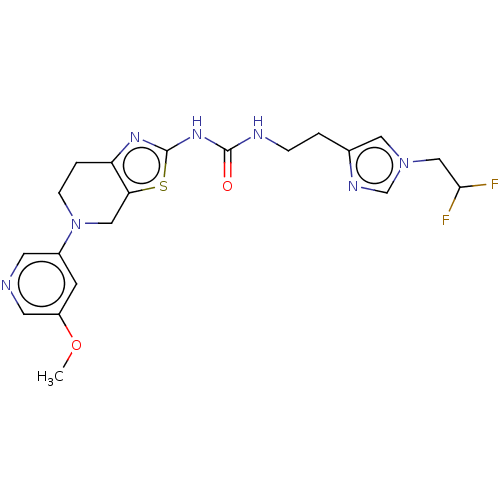
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
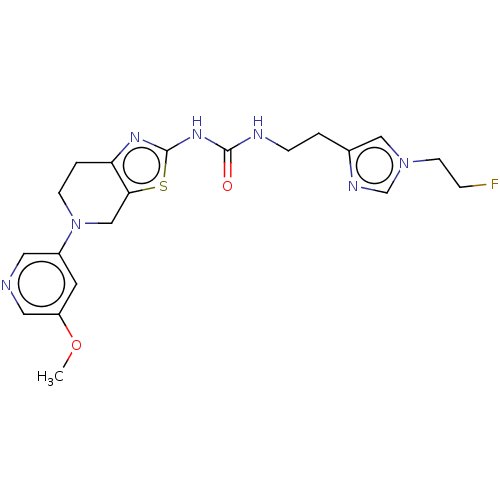
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109062
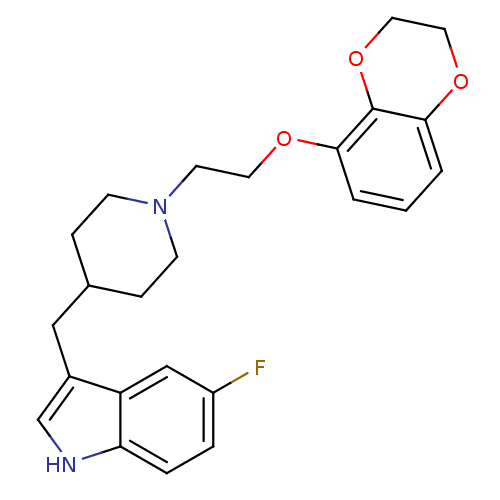
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCOc4cccc5OCCOc45)CC3)c2c1 Show InChI InChI=1S/C24H27FN2O3/c25-19-4-5-21-20(15-19)18(16-26-21)14-17-6-8-27(9-7-17)10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-5,15-17,26H,6-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
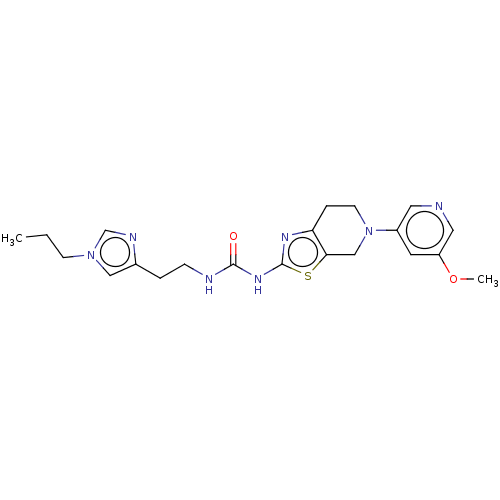
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166
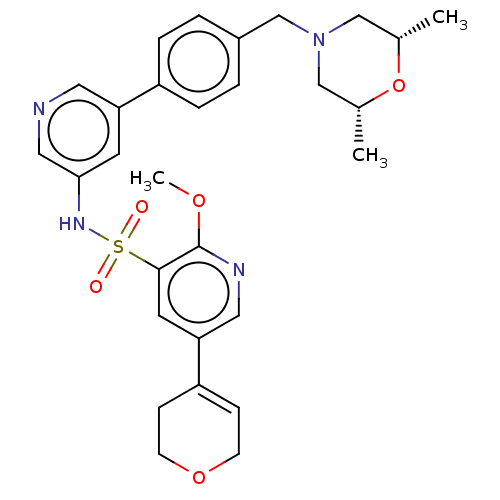
(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157
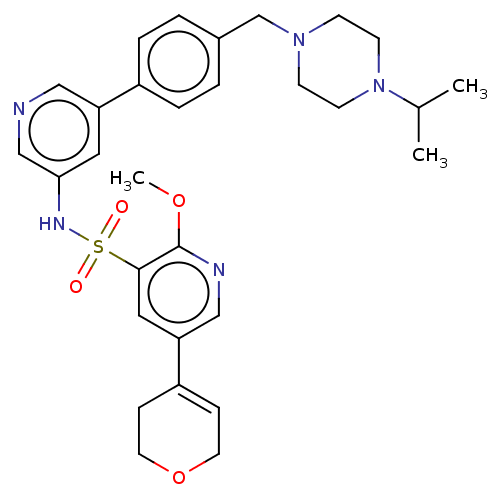
(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
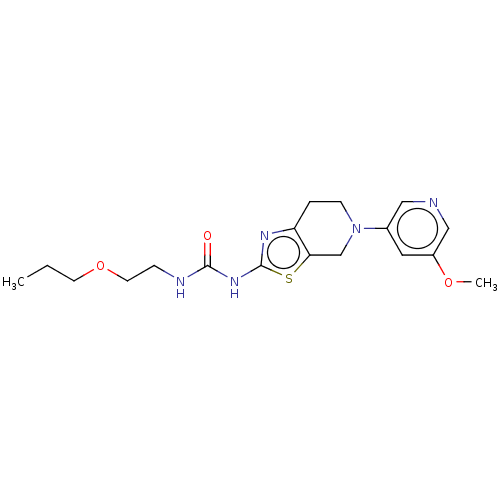
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573177

(CHEMBL4167702)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093437
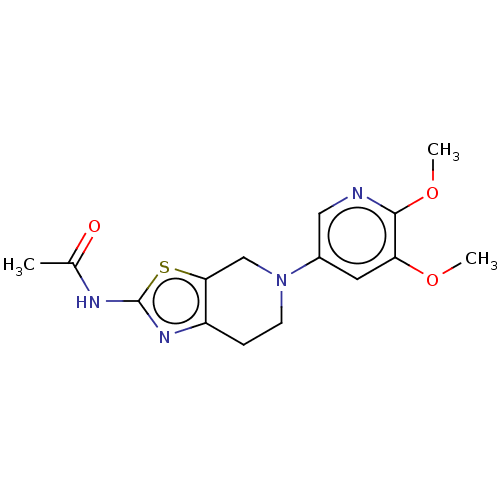
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573167
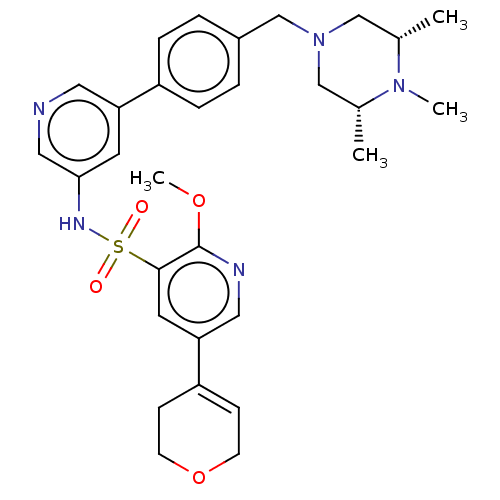
(CHEMBL4858875)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)N(C)[C@H](C)C2)cc1)C1=CCOCC1 |r,t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
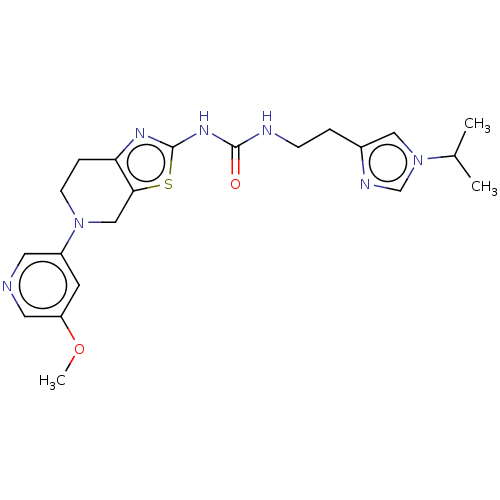
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
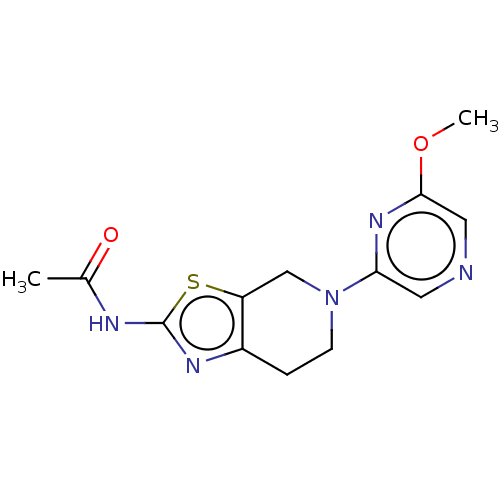
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50565986

(CHEMBL4789639)Show SMILES [2H]C([2H])([2H])NC(=O)c1cnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncc(F)cn2)c1OC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TYK2 JH2 domain (unknown origin) by HTRF assay based Morrison titration analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01698
BindingDB Entry DOI: 10.7270/Q2XW4PJX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093436
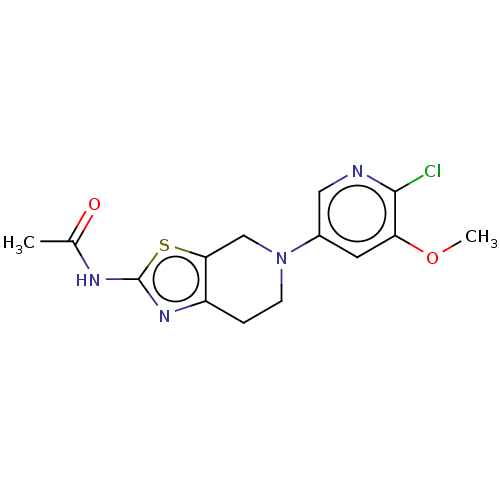
(CHEMBL3586669)Show InChI InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/p-2/b7-3+,8-4+/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093353
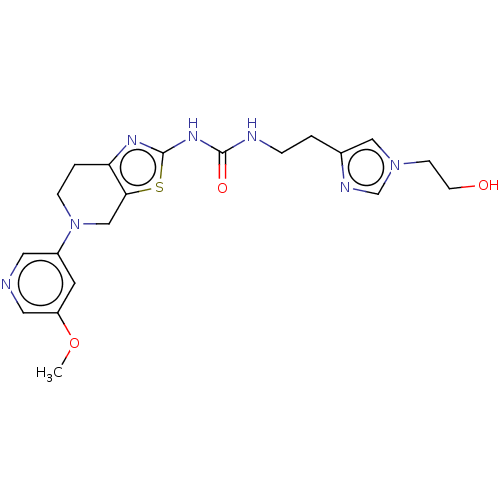
(CHEMBL3586680)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCO)cn3)sc2C1 Show InChI InChI=1S/C20H25N7O3S/c1-30-16-8-15(9-21-10-16)27-5-3-17-18(12-27)31-20(24-17)25-19(29)22-4-2-14-11-26(6-7-28)13-23-14/h8-11,13,28H,2-7,12H2,1H3,(H2,22,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573181
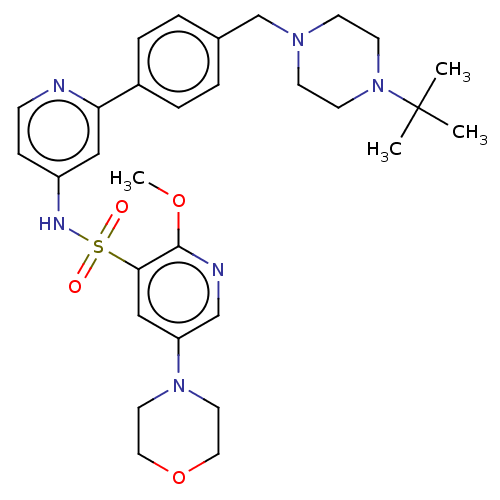
(CHEMBL4165185)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573164

(CHEMBL4873390)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)nc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573182
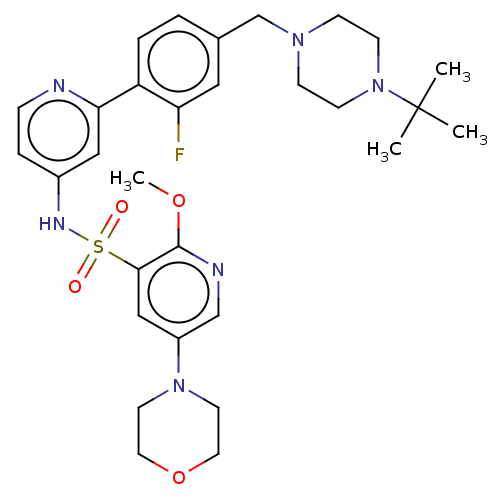
(CHEMBL4175571)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109058

(1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C23H23N3O/c1-2-5-21-18(4-1)20(16-25-21)17-9-12-26(13-10-17)14-15-27-23-7-3-6-22-19(23)8-11-24-22/h1-9,11,16,24-25H,10,12-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 134-42 (1995)
BindingDB Entry DOI: 10.7270/Q2ZC81CG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573180
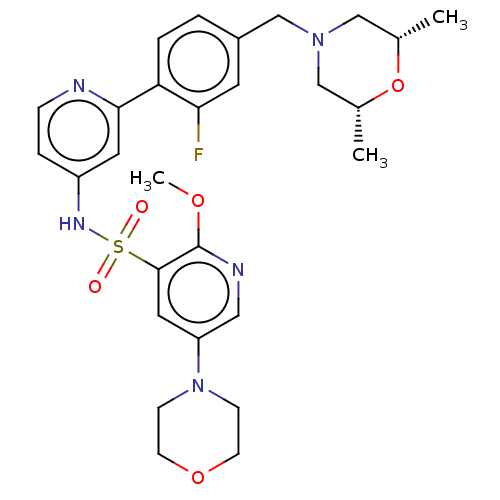
(CHEMBL4175737)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1F)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093391
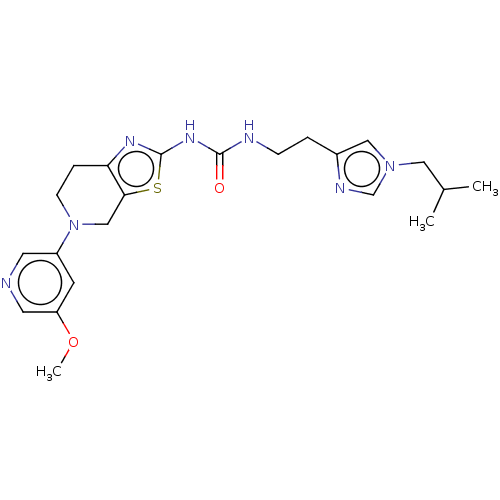
(CHEMBL3586675)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(C)C)cn3)sc2C1 Show InChI InChI=1S/C22H29N7O2S/c1-15(2)11-28-12-16(25-14-28)4-6-24-21(30)27-22-26-19-5-7-29(13-20(19)32-22)17-8-18(31-3)10-23-9-17/h8-10,12,14-15H,4-7,11,13H2,1-3H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573175
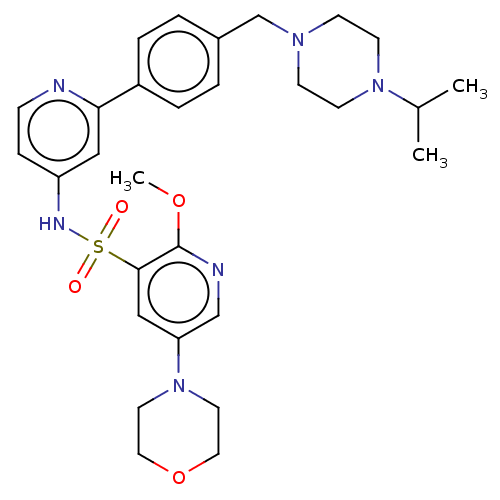
(CHEMBL4169192)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093439
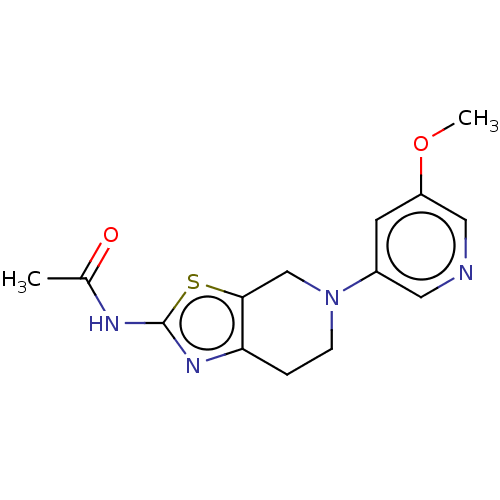
(CHEMBL3586666)Show InChI InChI=1S/C19H30O2/c1-18-7-5-12(20)9-11(18)3-4-13-14(18)6-8-19(2)15(13)10-16-17(19)21-16/h11-17,20H,3-10H2,1-2H3/t11-,12+,13+,14-,15-,16+,17+,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573179
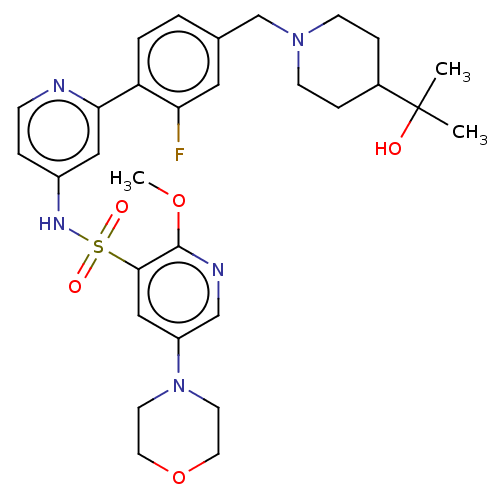
(CHEMBL4174874)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCC(CC2)C(C)(C)O)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573176

(CHEMBL4173087)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)c(F)c1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573178
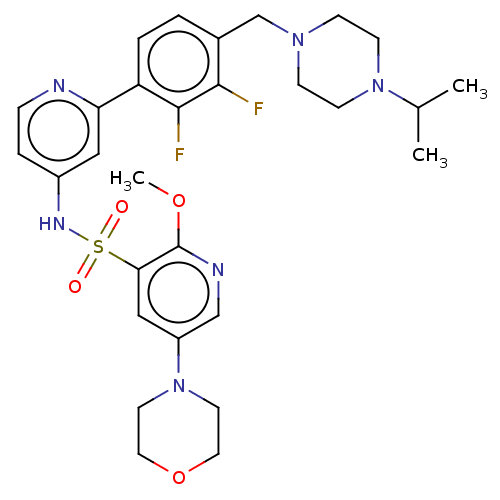
(CHEMBL4170075)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)c(F)c1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093419
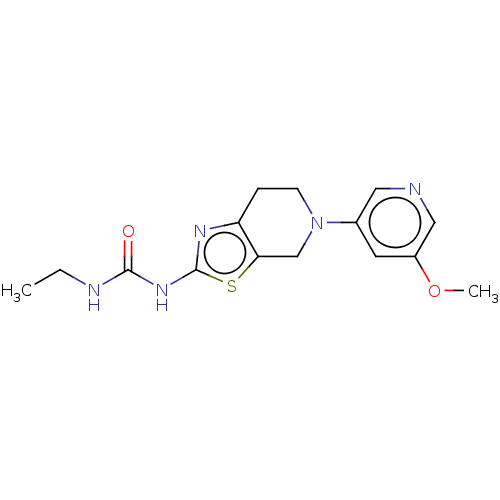
(CHEMBL3586671)Show InChI InChI=1S/C15H19N5O2S/c1-3-17-14(21)19-15-18-12-4-5-20(9-13(12)23-15)10-6-11(22-2)8-16-7-10/h6-8H,3-5,9H2,1-2H3,(H2,17,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 134-42 (1995)
BindingDB Entry DOI: 10.7270/Q2ZC81CG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582801
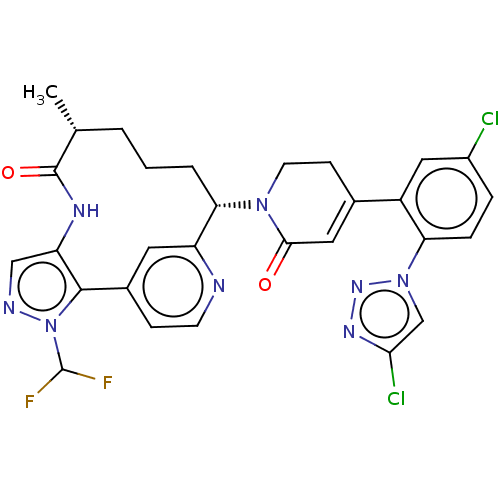
(CHEMBL5076656)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2cc(Cl)nn2)c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Receptor activity-modifying protein 1
(PIG) | BDBM85836
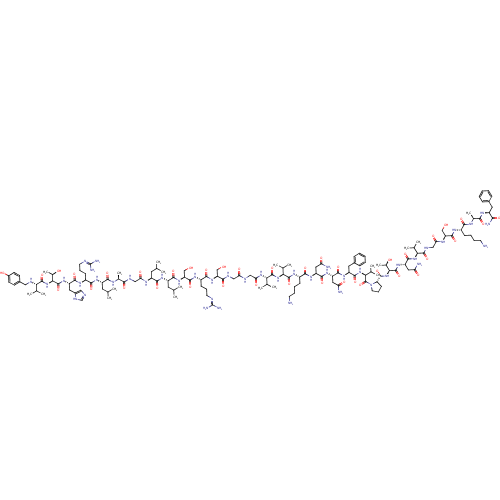
([N-benzol]h alpha-CGRP8-37)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NCc1ccc(O)cc1)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:173.178,63.66,172.175,147.148,131.132,122.123,204.207,108.109,69.70,17.21,25.32,83.84,218.221,50.51,wD:158.160,179.182,187.190,139.140,198.201,115.116,4.4,77.78,36.43,94.95,46.47,168.172,213.217,12.12,(77.08,2.16,;77.08,.62,;75.75,-.15,;78.41,-.15,;78.41,-1.69,;79.75,-2.46,;81.08,-1.69,;81.08,-.15,;82.42,-2.46,;83.75,-1.69,;85.08,-2.46,;85.08,-4,;86.42,-1.69,;86.42,-.15,;87.75,-2.46,;89.08,-1.69,;89.08,-.15,;90.42,-2.46,;90.42,-4,;91.75,-4.77,;91.75,-6.31,;93.08,-4,;91.75,-1.69,;91.75,-.15,;90.42,.62,;93.08,.62,;94.42,-.15,;94.42,-1.69,;95.75,-2.46,;95.75,-4,;97.09,-4.77,;97.09,-6.31,;98.42,-4,;93.08,2.16,;94.42,2.93,;95.75,2.16,;94.42,4.47,;93.08,5.24,;93.08,6.79,;91.84,7.69,;92.31,9.15,;93.85,9.15,;94.33,7.69,;95.75,5.24,;97.09,4.47,;97.09,2.93,;98.42,5.24,;99.75,4.47,;101.09,5.24,;101.09,6.79,;102.42,4.47,;103.75,5.24,;103.75,6.78,;105.09,7.55,;105.09,9.1,;106.42,9.87,;107.76,9.1,;109.09,9.87,;107.76,7.55,;106.42,6.78,;102.42,2.93,;103.75,2.16,;101.09,2.16,;98.42,6.79,;97.09,7.56,;99.75,7.56,;77.08,-2.46,;75.75,-1.69,;77.08,-4,;75.75,-4.77,;75.75,-6.31,;77.08,-7.08,;77.08,-8.62,;78.41,-6.31,;74.41,-4,;74.41,-2.46,;73.08,-4.77,;71.75,-4,;71.75,-2.46,;73.08,-1.69,;70.41,-4.77,;70.41,-6.31,;69.08,-4,;67.74,-4.77,;67.74,-6.31,;69.08,-7.08,;69.08,-8.62,;70.41,-9.39,;70.41,-10.93,;71.75,-11.7,;69.08,-11.7,;66.41,-4,;66.41,-2.46,;65.08,-4.77,;63.74,-4,;63.74,-2.46,;65.08,-1.69,;62.41,-4.77,;62.41,-6.31,;61.08,-4,;59.74,-4.77,;58.41,-4,;58.41,-2.46,;57.07,-4.77,;55.74,-4,;54.41,-4.77,;54.41,-6.31,;53.07,-4,;51.74,-4.77,;51.74,-6.31,;53.07,-7.08,;50.41,-7.08,;50.41,-4,;50.41,-2.46,;49.07,-4.77,;47.74,-4,;47.74,-2.46,;49.07,-1.69,;46.41,-1.69,;46.41,-4.77,;46.41,-6.31,;45.07,-4,;43.74,-4.77,;43.74,-6.31,;45.07,-7.08,;45.07,-8.62,;46.41,-9.39,;46.41,-10.93,;42.4,-4,;42.4,-2.46,;41.07,-4.77,;39.74,-4,;38.4,-4.77,;38.4,-6.31,;37.07,-7.08,;39.74,-7.08,;39.74,-2.46,;41.07,-1.69,;38.4,-1.69,;38.4,-.15,;39.74,.62,;39.74,2.16,;41.07,2.93,;38.4,2.93,;37.07,.62,;37.07,2.16,;35.74,-.15,;34.4,.62,;33.07,-.15,;33.07,-1.69,;31.73,-2.46,;31.73,-4,;33.07,-4.77,;34.4,-4,;34.4,-2.46,;34.4,2.16,;35.74,2.93,;33.07,2.93,;33.07,4.47,;34.4,5.24,;34.4,6.79,;35.74,4.47,;31.73,5.24,;30.4,4.47,;31.73,6.79,;32.98,7.69,;32.5,9.15,;30.96,9.15,;30.49,7.69,;29.02,7.21,;28.7,5.71,;27.88,8.24,;26.41,7.77,;26.09,6.26,;27.24,5.23,;24.63,5.79,;25.27,8.8,;25.59,10.31,;23.81,8.32,;22.66,9.35,;22.98,10.86,;21.84,11.89,;22.16,13.4,;20.37,11.42,;21.2,8.88,;20.05,9.91,;20.88,7.37,;19.41,6.9,;18.27,7.93,;16.8,7.45,;18.59,9.43,;19.09,5.39,;20.24,4.36,;17.63,4.91,;17.31,3.41,;15.84,2.93,;14.7,3.96,;15.52,1.42,;14.06,.95,;12.91,1.98,;11.45,1.5,;13.74,-.56,;14.88,-1.59,;12.27,-1.03,;11.95,-2.54,;13.1,-3.57,;12.78,-5.08,;13.92,-6.11,;13.6,-7.61,;14.74,-8.64,;10.49,-3.02,;9.34,-1.99,;10.17,-4.52,;8.7,-5,;7.56,-3.97,;8.38,-6.5,;9.53,-7.53,;6.92,-6.98,;6.6,-8.49,;7.74,-9.52,;7.42,-11.02,;5.96,-11.5,;5.64,-13.01,;6.78,-14.04,;8.25,-13.56,;8.57,-12.05,;5.13,-8.96,;3.99,-7.93,;4.81,-10.47,)| Show InChI InChI=1S/C146H236N44O39/c1-71(2)52-93(168-109(201)65-162-120(205)79(17)166-127(212)94(53-72(3)4)175-125(210)90(40-31-49-158-145(153)154)170-130(215)97(57-86-62-157-70-165-86)180-143(228)118(82(20)195)189-139(224)112(74(7)8)161-61-85-43-45-87(196)46-44-85)128(213)176-95(54-73(5)6)129(214)183-103(69-193)136(221)172-91(41-32-50-159-146(155)156)126(211)182-101(67-191)122(207)163-63-108(200)160-64-111(203)184-114(76(11)12)141(226)186-115(77(13)14)140(225)173-89(39-28-30-48-148)124(209)178-98(58-105(149)197)132(217)179-99(59-106(150)198)131(216)177-96(56-84-36-25-22-26-37-84)133(218)187-116(78(15)16)144(229)190-51-33-42-104(190)137(222)188-117(81(19)194)142(227)181-100(60-107(151)199)134(219)185-113(75(9)10)138(223)164-66-110(202)169-102(68-192)135(220)171-88(38-27-29-47-147)123(208)167-80(18)121(206)174-92(119(152)204)55-83-34-23-21-24-35-83/h21-26,34-37,43-46,62,70-82,88-104,112-118,161,191-196H,27-33,38-42,47-61,63-69,147-148H2,1-20H3,(H2,149,197)(H2,150,198)(H2,151,199)(H2,152,204)(H,157,165)(H,160,200)(H,162,205)(H,163,207)(H,164,223)(H,166,212)(H,167,208)(H,168,201)(H,169,202)(H,170,215)(H,171,220)(H,172,221)(H,173,225)(H,174,206)(H,175,210)(H,176,213)(H,177,216)(H,178,209)(H,179,217)(H,180,228)(H,181,227)(H,182,211)(H,183,214)(H,184,203)(H,185,219)(H,186,226)(H,187,218)(H,188,222)(H,189,224)(H4,153,154,158)(H4,155,156,159)/t79-,80-,81+,82+,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,112-,113-,114-,115-,116-,117-,118-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 1086-94 (2001)
BindingDB Entry DOI: 10.7270/Q29C6W07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573170
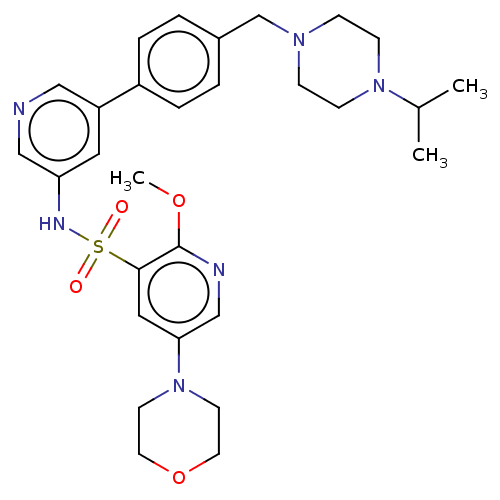
(CHEMBL4855234)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8336
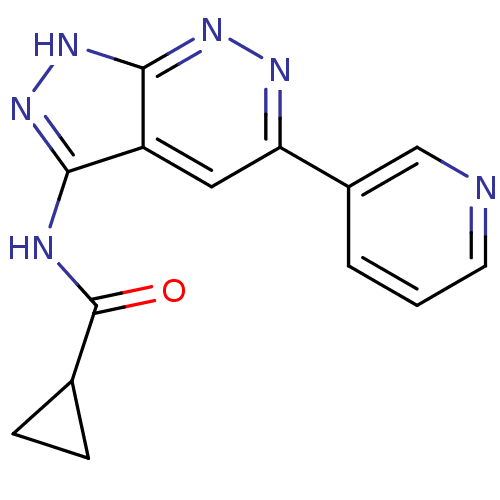
(N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...)Show InChI InChI=1S/C14H12N6O/c21-14(8-3-4-8)16-12-10-6-11(9-2-1-5-15-7-9)17-19-13(10)20-18-12/h1-2,5-8H,3-4H2,(H2,16,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Receptor activity-modifying protein 1
(PIG) | BDBM85837
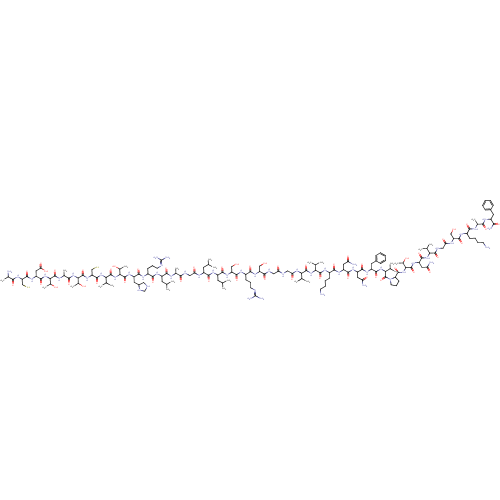
(h alpha-CGRP)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#6])-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-1-[#6]-[#7]-[#6]-[#7]-1)-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#16])-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6])-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#16])-[#7]-[#6](=O)-[#6](-[#6])-[#7])-[#6](-[#6])-[#8])-[#6](-[#6])-[#8])-[#6](-[#6])-[#6])-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](-[#7])=O Show InChI InChI=1S/C163H273N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,73-88,91-111,120-128,175,182,215-221,264-265H,31-37,42-72,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 1086-94 (2001)
BindingDB Entry DOI: 10.7270/Q29C6W07 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150101
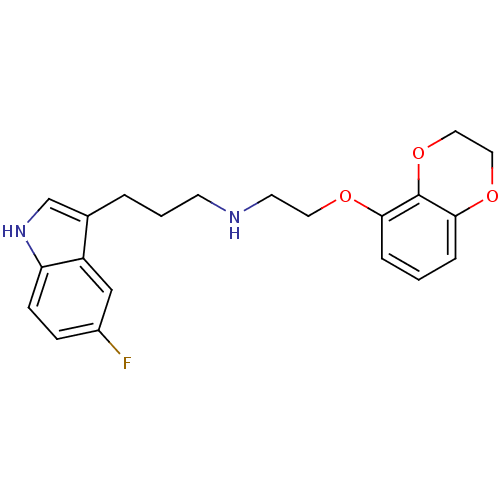
(CHEMBL419240 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show InChI InChI=1S/C21H23FN2O3/c22-16-6-7-18-17(13-16)15(14-24-18)3-2-8-23-9-10-25-19-4-1-5-20-21(19)27-12-11-26-20/h1,4-7,13-14,23-24H,2-3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165935
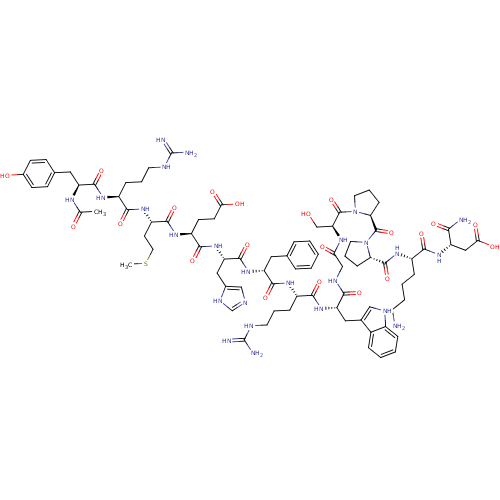
(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165931
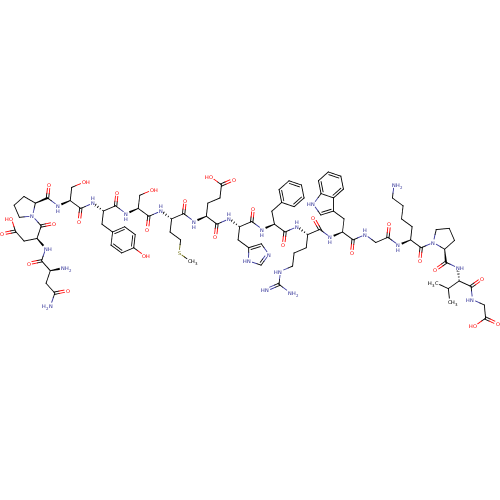
(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50102006
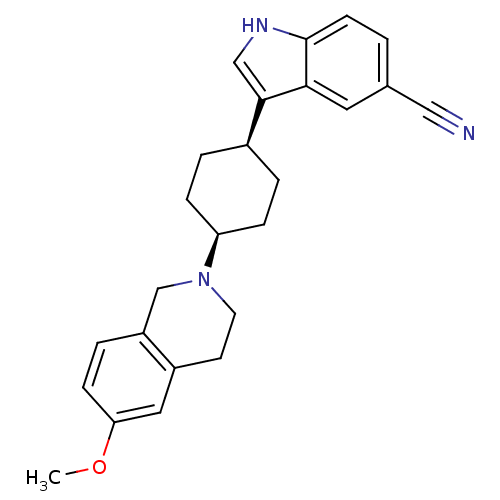
(3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...)Show SMILES COc1ccc2CN(CCc2c1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(cc12)C#N |r,wU:12.13,15.20,(10.58,5.27,;9.25,6.04,;7.91,5.27,;7.91,3.73,;6.57,2.97,;5.25,3.74,;3.93,2.97,;2.59,3.75,;2.59,5.28,;3.93,6.05,;5.26,5.28,;6.59,6.04,;1.26,2.98,;1.26,1.44,;-.07,.67,;-1.41,1.45,;-1.41,2.97,;-.08,3.75,;-2.74,.67,;-2.89,-.87,;-4.41,-1.2,;-5.19,.14,;-6.69,.45,;-7.18,1.91,;-6.15,3.07,;-4.64,2.76,;-4.16,1.3,;-6.63,4.53,;-7.11,5.99,)| Show InChI InChI=1S/C25H27N3O/c1-29-22-8-5-20-16-28(11-10-19(20)13-22)21-6-3-18(4-7-21)24-15-27-25-9-2-17(14-26)12-23(24)25/h2,5,8-9,12-13,15,18,21,27H,3-4,6-7,10-11,16H2,1H3/t18-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes |
Bioorg Med Chem Lett 11: 1885-8 (2001)
BindingDB Entry DOI: 10.7270/Q2GM86K9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50191633

((3S)-3-{(cyclopropylmethyl)[3-(5-fluoro-1H-indol-3...)Show SMILES NC(=O)c1ccc(F)c2OC[C@H](Cc12)N(CCCc1c[nH]c2ccc(F)cc12)CC1CC1 Show InChI InChI=1S/C25H27F2N3O2/c26-17-5-8-23-20(10-17)16(12-29-23)2-1-9-30(13-15-3-4-15)18-11-21-19(25(28)31)6-7-22(27)24(21)32-14-18/h5-8,10,12,15,18,29H,1-4,9,11,13-14H2,(H2,28,31)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
J Med Chem 49: 4785-9 (2006)
Article DOI: 10.1021/jm060218h
BindingDB Entry DOI: 10.7270/Q261114D |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284
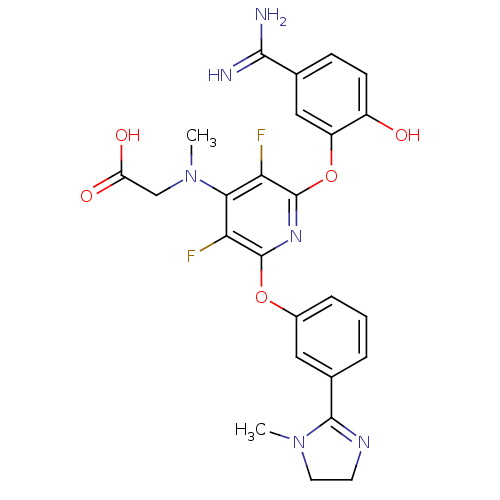
(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(PIG) | BDBM85837

(h alpha-CGRP)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#6])-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-1-[#6]-[#7]-[#6]-[#7]-1)-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#16])-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6])-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#16])-[#7]-[#6](=O)-[#6](-[#6])-[#7])-[#6](-[#6])-[#8])-[#6](-[#6])-[#8])-[#6](-[#6])-[#6])-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](-[#7])=O Show InChI InChI=1S/C163H273N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,73-88,91-111,120-128,175,182,215-221,264-265H,31-37,42-72,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 1086-94 (2001)
BindingDB Entry DOI: 10.7270/Q29C6W07 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8337

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES Fc1cccc(c1F)-c1cc2c(NC(=O)C3CCCC3)n[nH]c2nn1 Show InChI InChI=1S/C17H15F2N5O/c18-12-7-3-6-10(14(12)19)13-8-11-15(22-24-16(11)23-21-13)20-17(25)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H2,20,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM247411
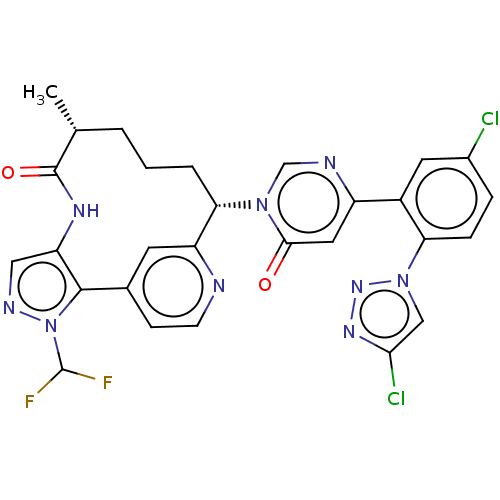
(US10336754, Example 353 | US11053247, Example 353 ...)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| Show InChI InChI=1S/C28H23Cl2F2N9O2/c1-15-3-2-4-23(20-9-16(7-8-33-20)26-21(36-27(15)43)12-35-41(26)28(31)32)39-14-34-19(11-25(39)42)18-10-17(29)5-6-22(18)40-13-24(30)37-38-40/h5-15,23,28H,2-4H2,1H3,(H,36,43)/t15-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B2 bradykinin receptor
(Cavia porcellus) | BDBM50406750

(Firazyr | HOE-140 | Icatibant)Show SMILES [H][C@]12C[C@H](N(C(=O)[C@H]3Cc4ccccc4CN3C(=O)[C@H](CO)NC(=O)[C@H](Cc3cccs3)NC(=O)CNC(=O)[C@@H]3C[C@@H](O)CN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](N)CCCNC(N)=N)[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. |
J Med Chem 36: 1450-60 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1QS1 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 134-42 (1995)
BindingDB Entry DOI: 10.7270/Q2ZC81CG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data