 Found 435 hits with Last Name = 'mcelligott' and Initial = 'dl'
Found 435 hits with Last Name = 'mcelligott' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27700
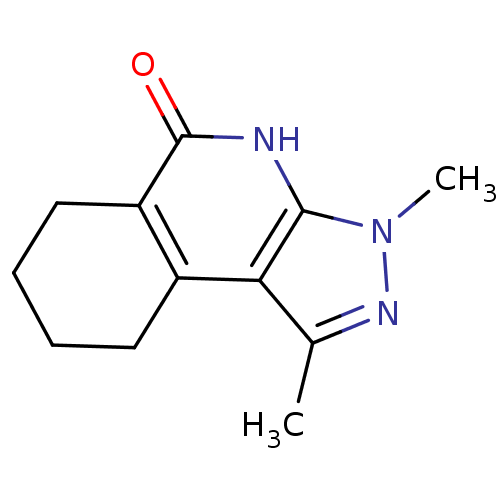
(3,5-dimethyl-4,5,7-triazatricyclo[7.4.0.0^{2,6}]tr...)Show InChI InChI=1S/C12H15N3O/c1-7-10-8-5-3-4-6-9(8)12(16)13-11(10)15(2)14-7/h3-6H2,1-2H3,(H,13,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27685

(2-{4-[(dimethylamino)methyl]phenyl}-3,10-diazatric...)Show SMILES CN(C)Cc1ccc(cc1)-c1[nH]c2cccc3C(=O)NCCc1c23 Show InChI InChI=1S/C20H21N3O/c1-23(2)12-13-6-8-14(9-7-13)19-15-10-11-21-20(24)16-4-3-5-17(22-19)18(15)16/h3-9,22H,10-12H2,1-2H3,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27702

(5-methyl-3-(thiophen-2-yl)-4,5,7-triazatricyclo[7....)Show InChI InChI=1S/C15H15N3OS/c1-18-14-12(13(17-18)11-7-4-8-20-11)9-5-2-3-6-10(9)15(19)16-14/h4,7-8H,2-3,5-6H2,1H3,(H,16,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27701

(5-methyl-3-phenyl-4,5,7-triazatricyclo[7.4.0.0^{2,...)Show InChI InChI=1S/C17H17N3O/c1-20-16-14(15(19-20)11-7-3-2-4-8-11)12-9-5-6-10-13(12)17(21)18-16/h2-4,7-8H,5-6,9-10H2,1H3,(H,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27497
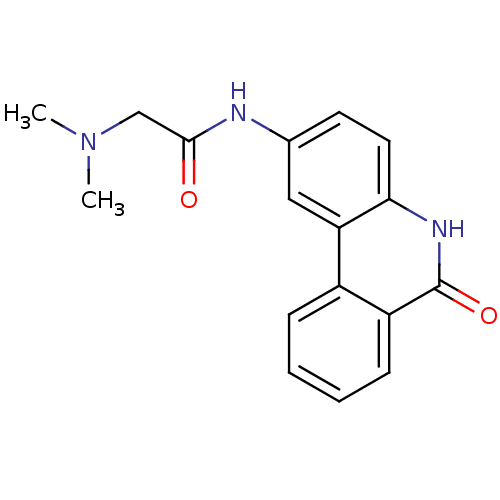
(2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...)Show InChI InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27694
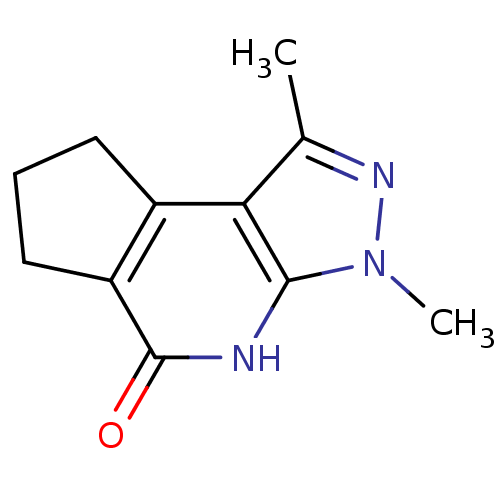
(3,5-dimethyl-4,5,7-triazatricyclo[7.3.0.0^{2,6}]do...)Show InChI InChI=1S/C11H13N3O/c1-6-9-7-4-3-5-8(7)11(15)12-10(9)14(2)13-6/h3-5H2,1-2H3,(H,12,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29.8 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27684
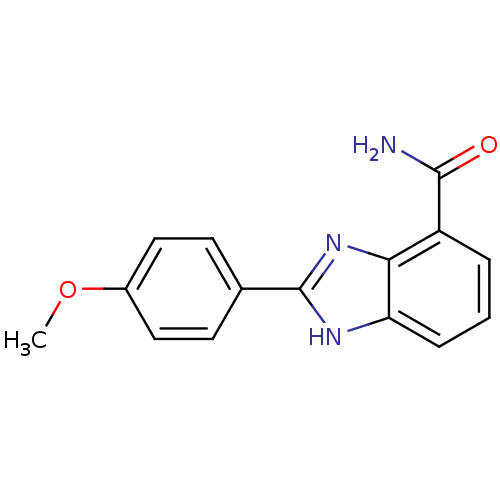
(2-(4-methoxyphenyl)-1H-1,3-benzodiazole-4-carboxam...)Show InChI InChI=1S/C15H13N3O2/c1-20-10-7-5-9(6-8-10)15-17-12-4-2-3-11(14(16)19)13(12)18-15/h2-8H,1H3,(H2,16,19)(H,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27689
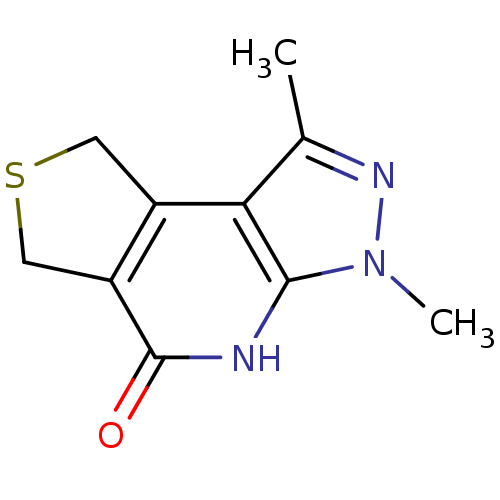
(3,5-dimethyl-11-thia-4,5,7-triazatricyclo[7.3.0.0^...)Show InChI InChI=1S/C10H11N3OS/c1-5-8-6-3-15-4-7(6)10(14)11-9(8)13(2)12-5/h3-4H2,1-2H3,(H,11,14) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92.7 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27695
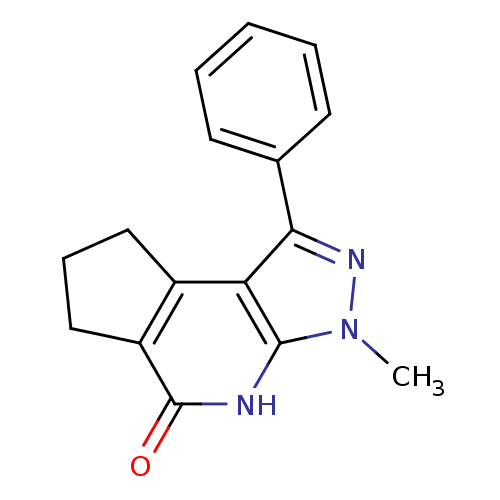
(5-methyl-3-phenyl-4,5,7-triazatricyclo[7.3.0.0^{2,...)Show InChI InChI=1S/C16H15N3O/c1-19-15-13(11-8-5-9-12(11)16(20)17-15)14(18-19)10-6-3-2-4-7-10/h2-4,6-7H,5,8-9H2,1H3,(H,17,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27690
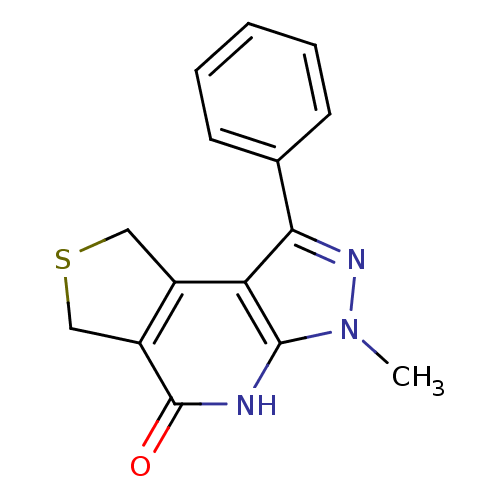
(5-methyl-3-phenyl-11-thia-4,5,7-triazatricyclo[7.3...)Show InChI InChI=1S/C15H13N3OS/c1-18-14-12(10-7-20-8-11(10)15(19)16-14)13(17-18)9-5-3-2-4-6-9/h2-6H,7-8H2,1H3,(H,16,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
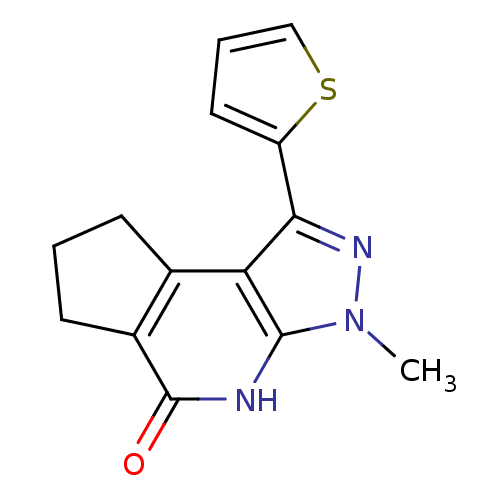
(Homo sapiens (Human)) | BDBM27696
(5-methyl-3-(thiophen-2-yl)-4,5,7-triazatricyclo[7....)Show InChI InChI=1S/C14H13N3OS/c1-17-13-11(12(16-17)10-6-3-7-19-10)8-4-2-5-9(8)14(18)15-13/h3,6-7H,2,4-5H2,1H3,(H,15,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27682
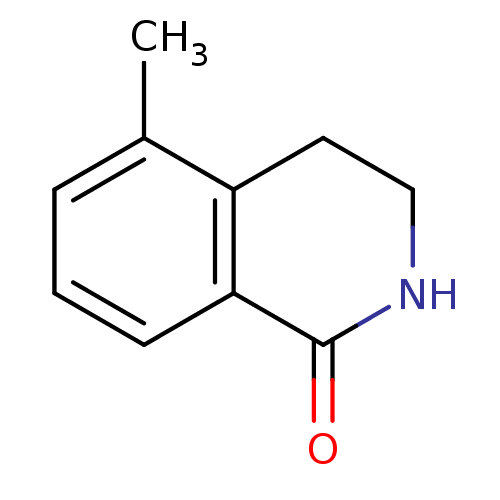
(5-methyl-1,2,3,4-tetrahydroisoquinolin-1-one | CHE...)Show InChI InChI=1S/C10H11NO/c1-7-3-2-4-9-8(7)5-6-11-10(9)12/h2-4H,5-6H2,1H3,(H,11,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27691

(5-methyl-3-(thiophen-2-yl)-11-thia-4,5,7-triazatri...)Show InChI InChI=1S/C13H11N3OS2/c1-16-12-10(11(15-16)9-3-2-4-19-9)7-5-18-6-8(7)13(17)14-12/h2-4H,5-6H2,1H3,(H,14,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 428 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602616
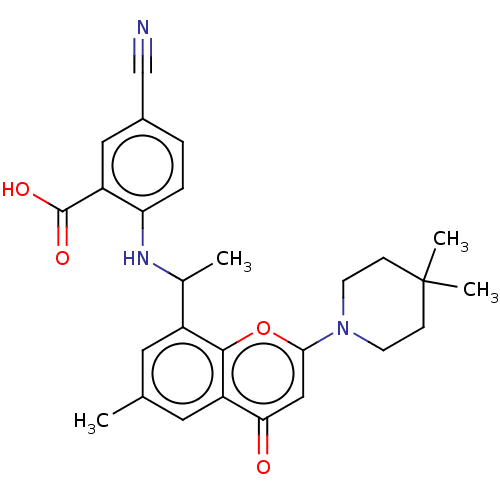
(5-Cyano-2-[1-[2-(4,4-dimethyl-1- piperidyl)-6-meth...)Show SMILES CC(Nc1ccc(cc1C(O)=O)C#N)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602619

(2-[1-[2-(4,4-Dimethyl-1-piperidyl)-6- methyl-4-oxo...)Show SMILES CONC(=O)c1ccccc1NC(C)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602620
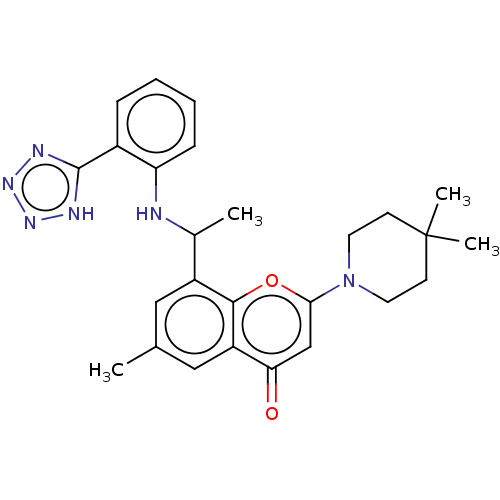
(2-(4,4-Dimethyl-1-piperidyl)-6-methyl- 8-[1-[2-(1H...)Show SMILES CC(Nc1ccccc1-c1nnn[nH]1)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602628
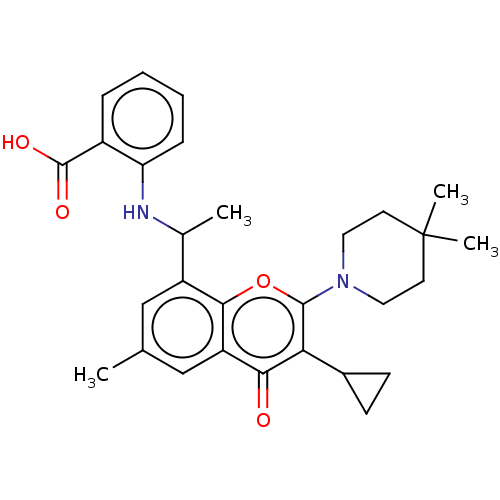
(2-[1-[3-Cyclopropyl-2-(4,4-dimethyl-1- piperidyl)-...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(C)(C)CC1)c(C1CC1)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602629
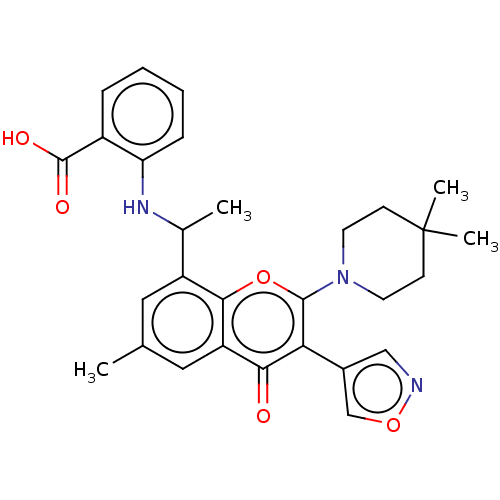
(2-[1-[2-(4,4-Dimethyl-1-piperidyl)-6- isoxazol-4-y...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(C)(C)CC1)c(-c1cnoc1)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602631

(2-[1-[2-(4,4-dimethyl-1-piperidyl)-3- ethyl-6-meth...)Show SMILES CCc1c(oc2c(cc(C)cc2c1=O)C(C)Nc1ccccc1C(O)=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602634

(2-[1-[2-(3-Chloroazetidin-1-yl)-6- methyl-4-oxo-ch...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC(Cl)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602636

(2-[1-[2-(3,3-Dimethyl-1-piperidyl)-6- methyl-4-oxo...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCCC(C)(C)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602640

(2-[1-[2-(Isobutylamino)-6-methyl-4- oxo-chromen-8-...)Show SMILES CC(C)CNc1cc(=O)c2cc(C)cc(C(C)Nc3ccccc3C(O)=O)c2o1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602646

(2-[1-[2-(2-Azaspiro[3.5]nonan-2-yl)- 6-methyl-4-ox...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC2(C1)CCCCC2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602651

(2-[1-[2-(5-Fluoroisoindolin-2-yl)-6- methyl-4-oxo-...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602651

(2-[1-[2-(5-Fluoroisoindolin-2-yl)-6- methyl-4-oxo-...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602666

(2-[1-[2-(4,4-Difluoro-1- piperidyl)-6-methyl-4-oxo...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC(F)(F)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602670

(2-[1-[2-(3,4-Dihydro-1H- pyrrolo[1,2-a]pyrazin-2-y...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCn2cccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602671
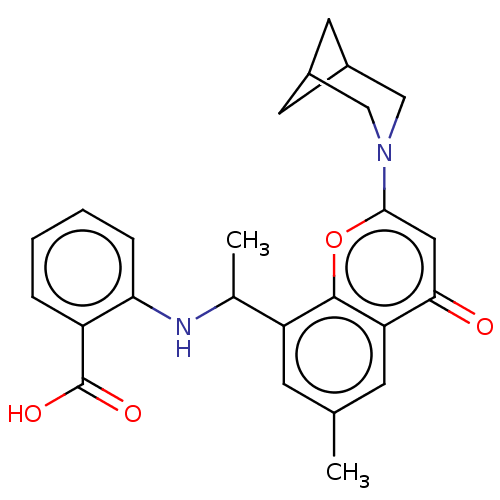
(2-[1-[2-(3- Azabicyclo[3.1.1]heptan-3-yl)- 6-methy...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC2CC(C2)C1 |(,-2.31,;-1.33,-1.54,;-2.67,-2.31,;-4,-1.54,;-4,,;-5.33,.77,;-6.67,,;-6.67,-1.54,;-5.33,-2.31,;-5.33,-3.85,;-6.67,-4.62,;-4,-4.62,;-1.33,,;-2.67,.77,;-2.67,2.31,;-4,3.08,;-1.33,3.08,;,2.31,;,.77,;1.33,,;2.67,.77,;2.67,2.31,;1.33,3.08,;1.33,4.62,;4,,;4,-1.54,;5.33,-2.31,;6.67,-1.54,;6.67,,;5.33,-.77,;5.33,.77,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602672
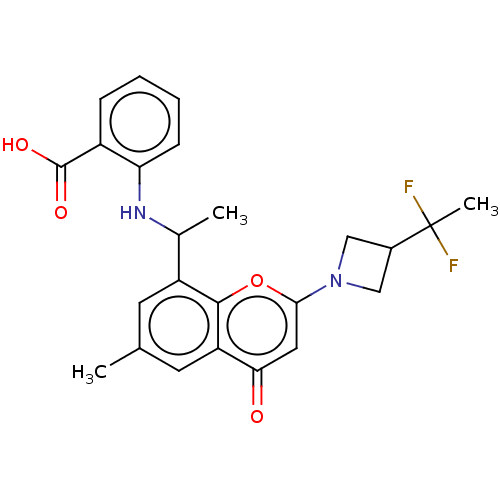
(2-[1-[2-[3-(1,1- Difluoroethyl)azetidin-l-yl]-6- m...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC(C1)C(C)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602674

(2-[1-[6-Methyl-4-oxo-2-(1- phenyl-1,6- diazaspiro[...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC2(CCN2c2ccccc2)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602675
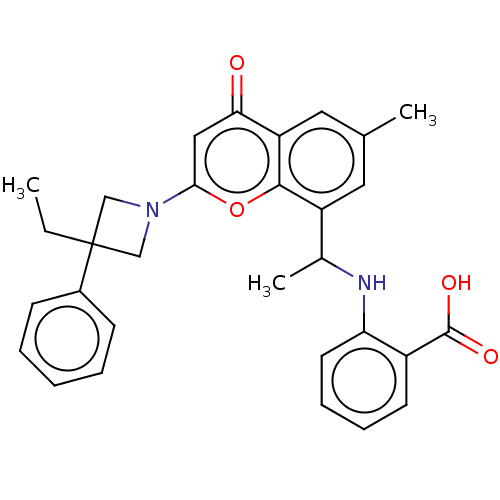
(2-[1-[2-(3-Ethyl-3-phenyl- azetidin-1-yl)-6-methyl...)Show SMILES CCC1(CN(C1)c1cc(=O)c2cc(C)cc(C(C)Nc3ccccc3C(O)=O)c2o1)c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602676
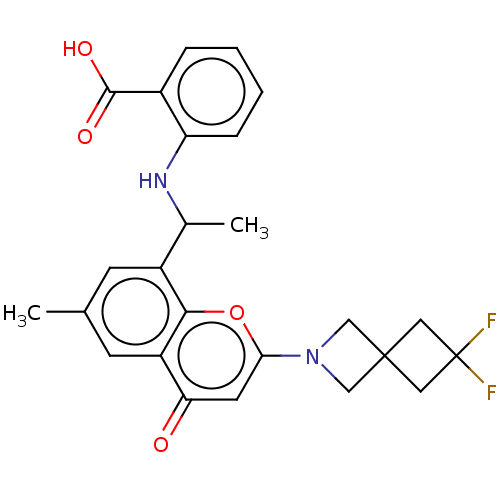
(2-[1[2-(6,6-Difluoro-2- azaspiro[3.3]heptan-2-yl)-...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC2(C1)CC(F)(F)C2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602677
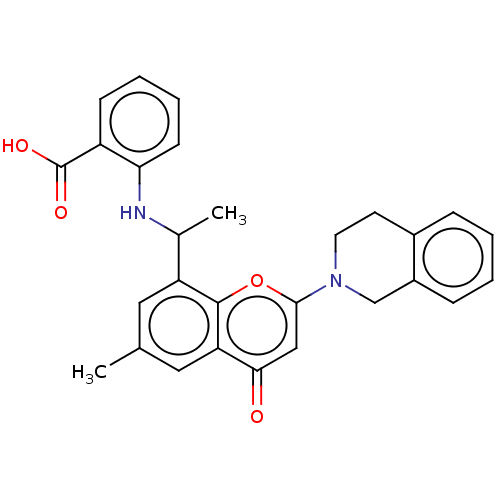
(2-[1-[2-(3,4-Dihydro-1H- isoquinolin-2-yl)-6-methy...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCc2ccccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602678
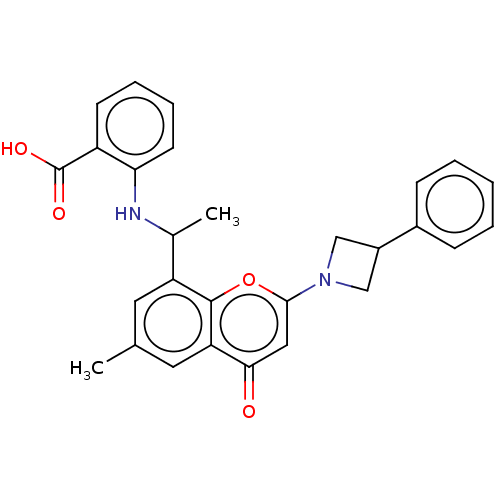
(US11649227, Example 236 | US20230286960, Example 2...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CC(C1)c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602682
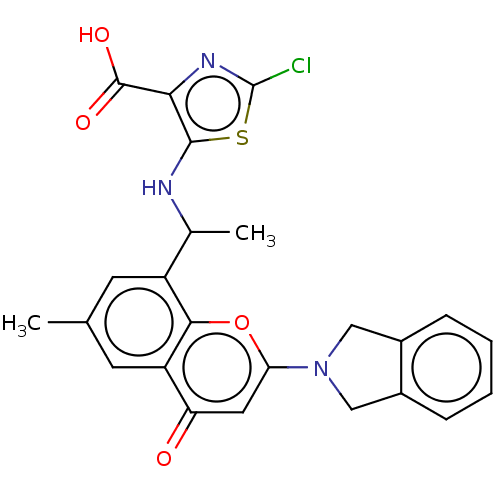
(2-Chloro-5-[1-(2-isoindolin-2-yl- 6-methyl-4-oxo-c...)Show SMILES CC(Nc1sc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602684
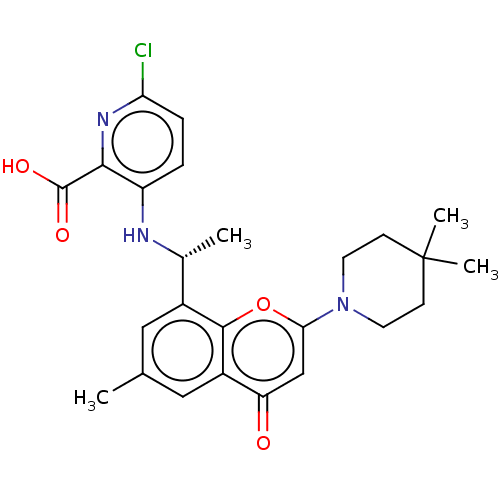
(6-Chloro-3-[[(1R)-1-[2-(4,4- dimethyl-1-piperidyl)...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602684

(6-Chloro-3-[[(1R)-1-[2-(4,4- dimethyl-1-piperidyl)...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602685

(2-[1-(2-Isoindolin-2-yl-3,6- dimethyl-4-oxo-chrome...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602686
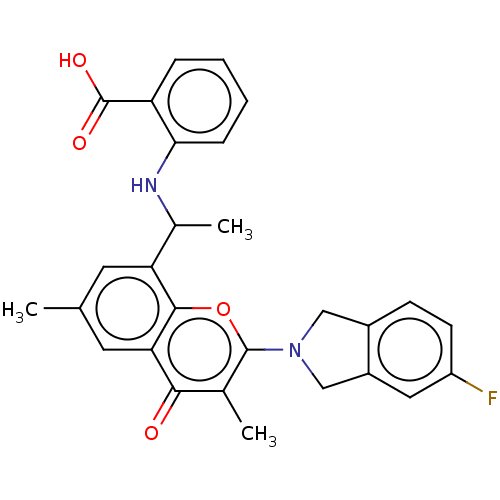
(US11649227, Example 272 | US20230286960, Example 2...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602687
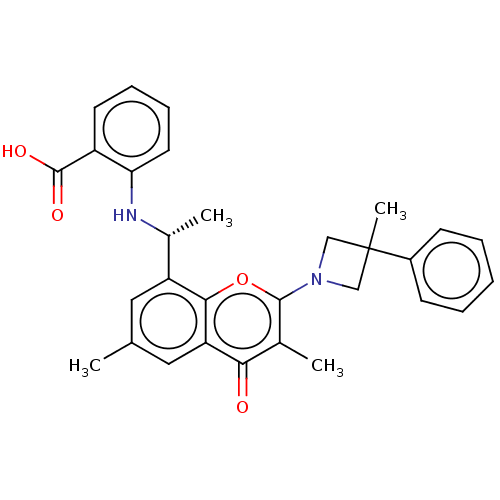
(US11649227, Example 274 | US20230286960, Example 2...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CC(C)(C1)c1ccccc1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602687

(US11649227, Example 274 | US20230286960, Example 2...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CC(C)(C1)c1ccccc1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602688
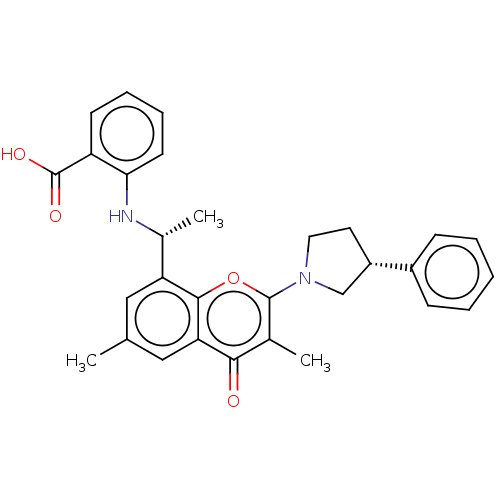
(2- [[(1R)-1- [3 ,6-dimethyl-4-oxo-2- [(3R)-3-pheny...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CC[C@@H](C1)c1ccccc1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602688

(2- [[(1R)-1- [3 ,6-dimethyl-4-oxo-2- [(3R)-3-pheny...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CC[C@@H](C1)c1ccccc1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602689
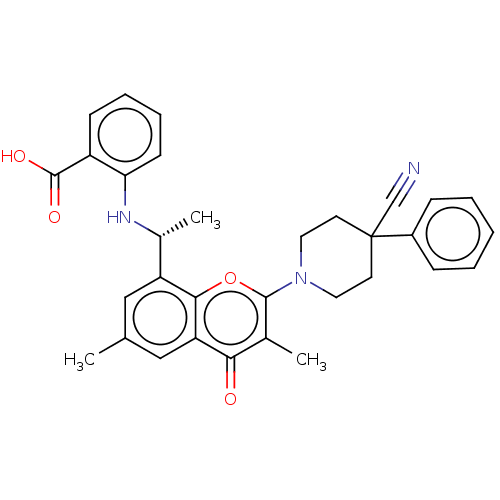
(US11649227, Example 288 | US20230286960, Example 2...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(CC1)(C#N)c1ccccc1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602690
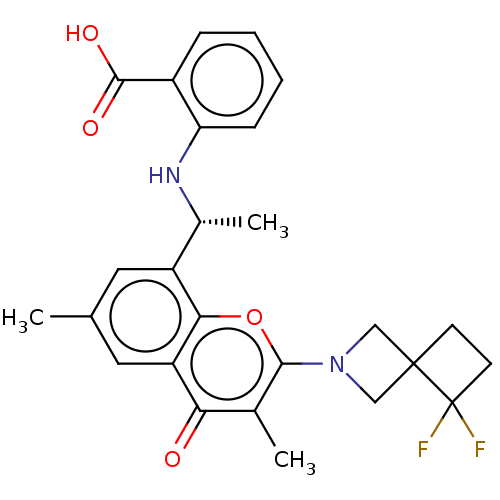
(US11649227, Example 289 | US20230286960, Example 2...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CC3(CCC3(F)F)C1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602692
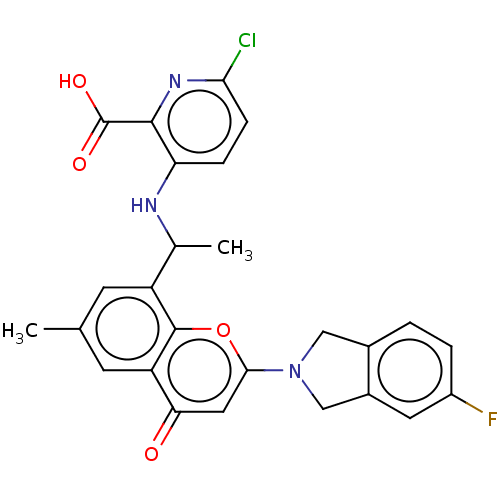
(US11649227, Example 297 | US20230286960, Example 2...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602693
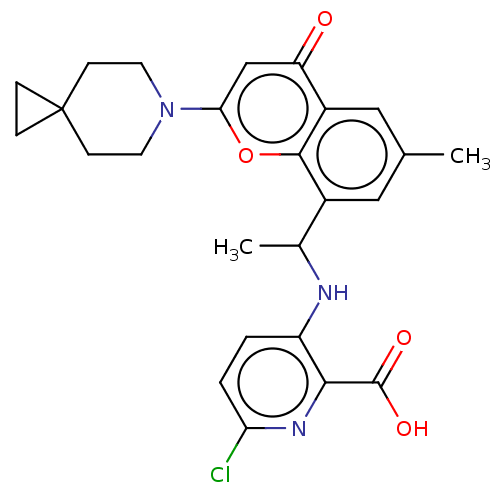
(US11649227, Example 298 | US20230286960, Example 2...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC2(CC2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602693

(US11649227, Example 298 | US20230286960, Example 2...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC2(CC2)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data