 Found 309 hits with Last Name = 'houston' and Initial = 'dr'
Found 309 hits with Last Name = 'houston' and Initial = 'dr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767
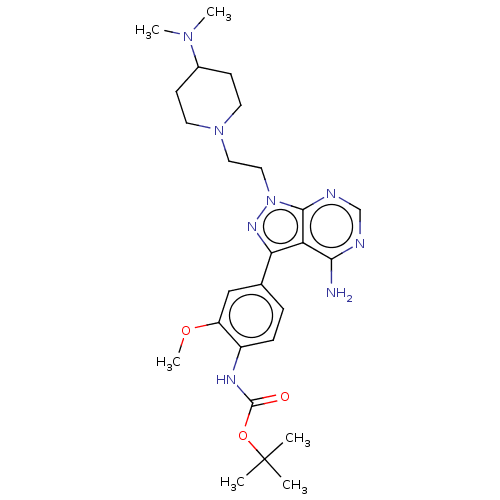
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as su... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10854
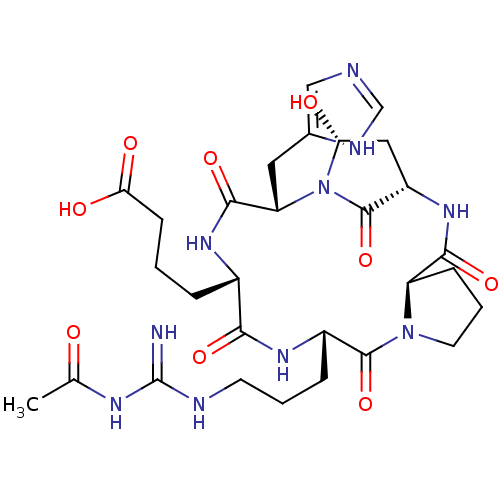
(4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...)Show SMILES CC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCC(O)=O)NC(=O)[C@H](Cc2cnc[nH]2)N2[C@H](O)C[C@H](NC(=O)[C@H]3CCCN3C1=O)C2=O |r| Show InChI InChI=1S/C29H42N10O9/c1-15(40)34-29(30)32-9-3-6-18-27(47)38-10-4-7-20(38)25(45)37-19-12-22(41)39(28(19)48)21(11-16-13-31-14-33-16)26(46)35-17(24(44)36-18)5-2-8-23(42)43/h13-14,17-22,41H,2-12H2,1H3,(H,31,33)(H,35,46)(H,36,44)(H,37,45)(H,42,43)(H3,30,32,34,40)/t17-,18-,19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10853
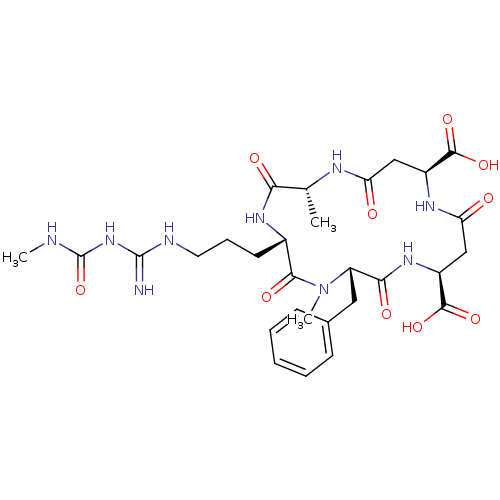
((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...)Show SMILES CNC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@@H](C)NC(=O)C[C@H](NC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H41N9O10/c1-15-23(41)35-17(10-7-11-32-28(30)37-29(48)31-2)25(43)38(3)20(12-16-8-5-4-6-9-16)24(42)36-19(27(46)47)14-22(40)34-18(26(44)45)13-21(39)33-15/h4-6,8-9,15,17-20H,7,10-14H2,1-3H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)(H,44,45)(H,46,47)(H4,30,31,32,37,48)/t15-,17+,18+,19+,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.30E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human ABL using EAIYAAPFAKKK as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184776
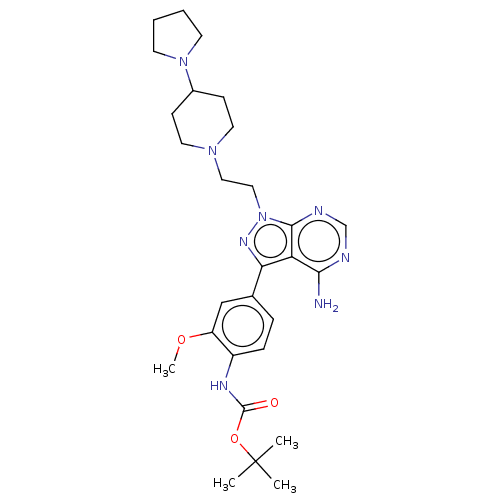
(CHEMBL3824233 | US10294227, Code 518)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C28H40N8O3/c1-28(2,3)39-27(37)32-21-8-7-19(17-22(21)38-4)24-23-25(29)30-18-31-26(23)36(33-24)16-15-34-13-9-20(10-14-34)35-11-5-6-12-35/h7-8,17-18,20H,5-6,9-16H2,1-4H3,(H,32,37)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184777
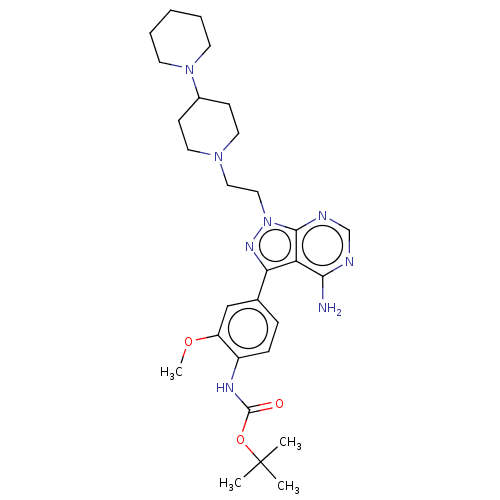
(CHEMBL3823104 | US10294227, Code 519)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C29H42N8O3/c1-29(2,3)40-28(38)33-22-9-8-20(18-23(22)39-4)25-24-26(30)31-19-32-27(24)37(34-25)17-16-35-14-10-21(11-15-35)36-12-6-5-7-13-36/h8-9,18-19,21H,5-7,10-17H2,1-4H3,(H,33,38)(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length AXL (R497 to Y821 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184785
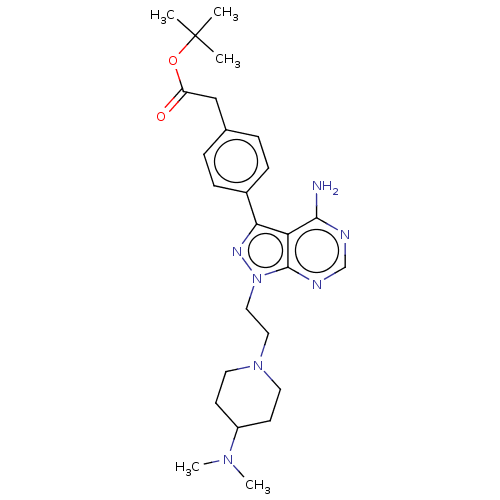
(CHEMBL3823620 | US10294227, Code 553)Show SMILES CN(C)C1CCN(CCn2nc(-c3ccc(CC(=O)OC(C)(C)C)cc3)c3c(N)ncnc23)CC1 Show InChI InChI=1S/C26H37N7O2/c1-26(2,3)35-21(34)16-18-6-8-19(9-7-18)23-22-24(27)28-17-29-25(22)33(30-23)15-14-32-12-10-20(11-13-32)31(4)5/h6-9,17,20H,10-16H2,1-5H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184786
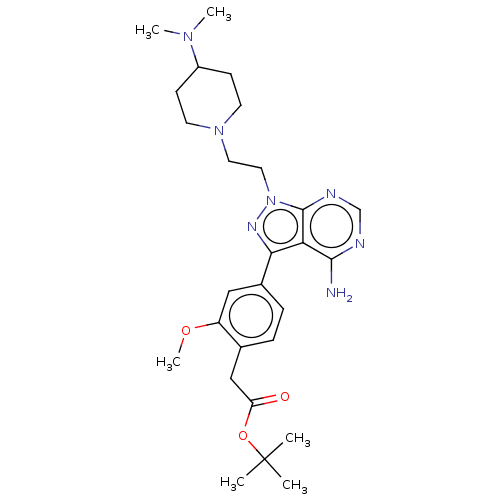
(CHEMBL3824116 | US10294227, Code 565)Show SMILES COc1cc(ccc1CC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C27H39N7O3/c1-27(2,3)37-22(35)16-18-7-8-19(15-21(18)36-6)24-23-25(28)29-17-30-26(23)34(31-24)14-13-33-11-9-20(10-12-33)32(4)5/h7-8,15,17,20H,9-14,16H2,1-6H3,(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MER (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184781
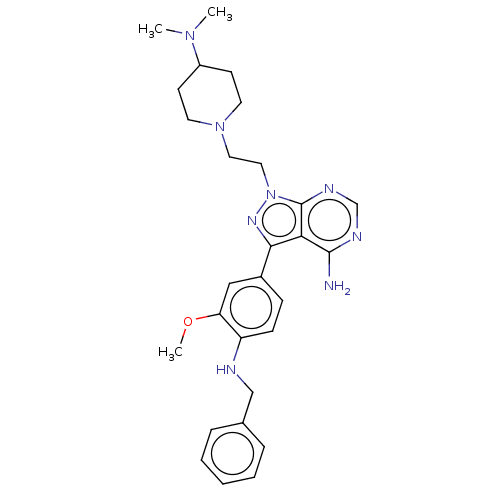
(CHEMBL3823543 | US10294227, Code 533)Show SMILES COc1cc(ccc1NCc1ccccc1)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C28H36N8O/c1-34(2)22-11-13-35(14-12-22)15-16-36-28-25(27(29)31-19-32-28)26(33-36)21-9-10-23(24(17-21)37-3)30-18-20-7-5-4-6-8-20/h4-10,17,19,22,30H,11-16,18H2,1-3H3,(H2,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length Aurora A (E122 to K401 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as substrate i... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length VEGFR2 (R787 to P1253 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRalpha using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM25116
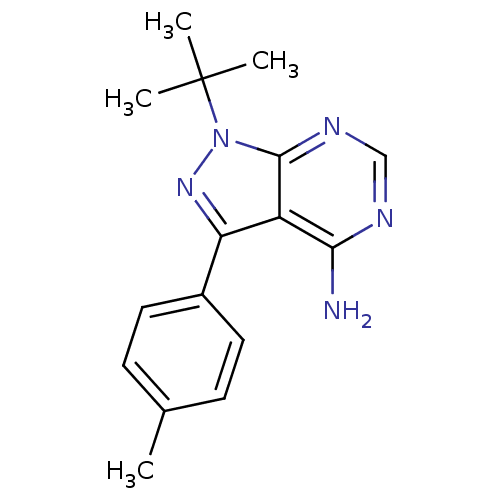
(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human RET using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50184766

(CHEMBL3823861 | US10294227, Code 221)Show SMILES CN(C)CC1CCN(CCn2nc(-c3cnc4[nH]ccc4c3)c3c(N)ncnc23)CC1 Show InChI InChI=1S/C22H29N9/c1-29(2)13-15-4-7-30(8-5-15)9-10-31-22-18(20(23)26-14-27-22)19(28-31)17-11-16-3-6-24-21(16)25-12-17/h3,6,11-12,14-15H,4-5,7-10,13H2,1-2H3,(H,24,25)(H2,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length AXL (R497 to Y821 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Homo sapiens (Human)) | BDBM10854

(4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...)Show SMILES CC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCC(O)=O)NC(=O)[C@H](Cc2cnc[nH]2)N2[C@H](O)C[C@H](NC(=O)[C@H]3CCCN3C1=O)C2=O |r| Show InChI InChI=1S/C29H42N10O9/c1-15(40)34-29(30)32-9-3-6-18-27(47)38-10-4-7-20(38)25(45)37-19-12-22(41)39(28(19)48)21(11-16-13-31-14-33-16)26(46)35-17(24(44)36-18)5-2-8-23(42)43/h13-14,17-22,41H,2-12H2,1H3,(H,31,33)(H,35,46)(H,36,44)(H,37,45)(H,42,43)(H3,30,32,34,40)/t17-,18-,19-,20+,21-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 5.2 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384600
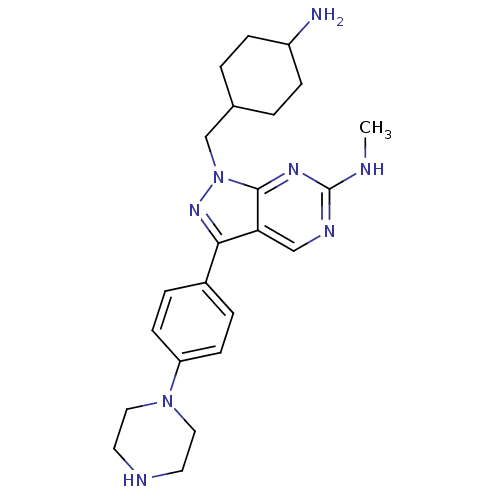
(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length MER (R557 to L884 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM25116

(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length TYRO3 (S497 to T814 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subs... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MER (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length DDR1 (R565 to V876 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length PDGFRA (V575 to D1002 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455348
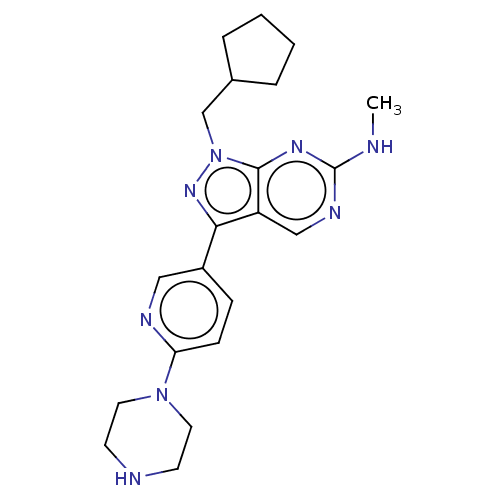
(CHEMBL4213003)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCNCC1 Show InChI InChI=1S/C21H28N8/c1-22-21-25-13-17-19(27-29(20(17)26-21)14-15-4-2-3-5-15)16-6-7-18(24-12-16)28-10-8-23-9-11-28/h6-7,12-13,15,23H,2-5,8-11,14H2,1H3,(H,22,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184775
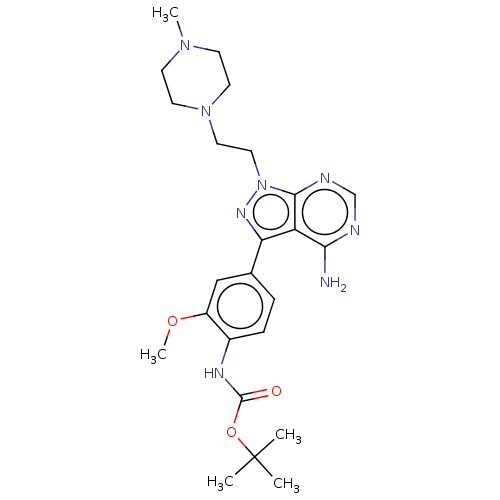
(CHEMBL3823451)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCN(C)CC2)c2ncnc(N)c12 Show InChI InChI=1S/C24H34N8O3/c1-24(2,3)35-23(33)28-17-7-6-16(14-18(17)34-5)20-19-21(25)26-15-27-22(19)32(29-20)13-12-31-10-8-30(4)9-11-31/h6-7,14-15H,8-13H2,1-5H3,(H,28,33)(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length Aurora A (E122 to K401 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as substrate i... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184766

(CHEMBL3823861 | US10294227, Code 221)Show SMILES CN(C)CC1CCN(CCn2nc(-c3cnc4[nH]ccc4c3)c3c(N)ncnc23)CC1 Show InChI InChI=1S/C22H29N9/c1-29(2)13-15-4-7-30(8-5-15)9-10-31-22-18(20(23)26-14-27-22)19(28-31)17-11-16-3-6-24-21(16)25-12-17/h3,6,11-12,14-15H,4-5,7-10,13H2,1-2H3,(H,24,25)(H2,23,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM25116

(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length Aurora A (E122 to K401 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as substrate i... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length DDR1 (R565 to V876 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM25116

(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data