 Found 36 hits with Last Name = 'yu' and Initial = 'dy'
Found 36 hits with Last Name = 'yu' and Initial = 'dy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060922

(CHEMBL3394769)Show SMILES [#8]-[#6]-c1ccc(-c2ccc(-[#8])cc2)c(\[#6](=[#6]-2/[#6]=[#6]-[#6](=O)-[#6]=[#6]-2)-c2ccc(-[#8])cc2)c1C#Cc1ccc(-[#8])cc1 |c:17,21| Show InChI InChI=1S/C34H24O5/c35-21-26-10-20-31(23-4-13-28(37)14-5-23)34(32(26)19-3-22-1-11-27(36)12-2-22)33(24-6-15-29(38)16-7-24)25-8-17-30(39)18-9-25/h1-2,4-18,20,35-38H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093525
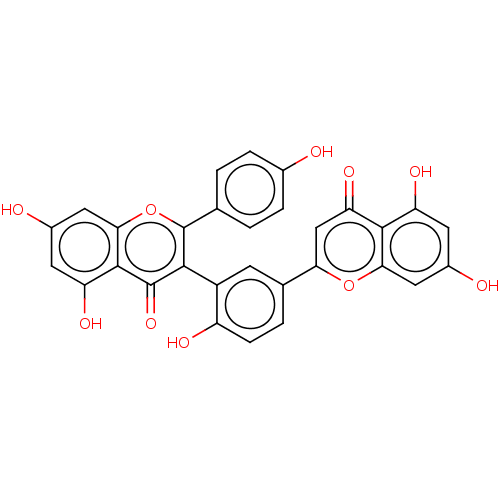
(taiwaniaflavone)Show SMILES Oc1ccc(cc1)-c1oc2cc(O)cc(O)c2c(=O)c1-c1cc(ccc1O)-c1cc(=O)c2c(O)cc(O)cc2o1 |(2.2,-12.35,;.87,-13.13,;.88,-14.67,;-.45,-15.44,;-1.79,-14.67,;-1.8,-13.14,;-.47,-12.36,;-3.12,-15.45,;-4.47,-14.67,;-5.81,-15.46,;-7.15,-14.7,;-8.47,-15.47,;-9.81,-14.7,;-8.48,-17.01,;-7.14,-17.78,;-7.15,-19.32,;-5.8,-17.02,;-4.46,-17.78,;-4.45,-19.32,;-3.11,-17,;-1.78,-17.77,;-.46,-17,;.88,-17.76,;.89,-19.3,;-.45,-20.08,;-1.78,-19.31,;-3.11,-20.08,;2.21,-16.98,;2.19,-15.44,;3.52,-14.67,;3.52,-13.13,;4.86,-15.43,;6.18,-14.67,;6.17,-13.13,;7.51,-15.42,;7.52,-16.96,;8.86,-17.73,;6.19,-17.73,;4.86,-16.97,;3.54,-17.75,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)30-26(29(38)28-21(36)9-17(33)11-25(28)40-30)18-7-14(3-6-19(18)34)23-12-22(37)27-20(35)8-16(32)10-24(27)39-23/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50259862
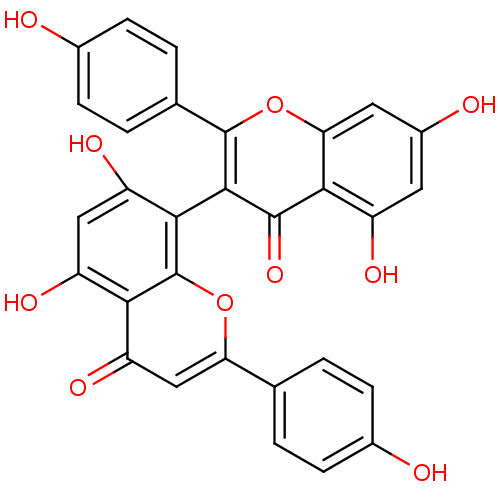
(13,II8-biapigenin | 3,8''-biapigenin | CHEMBL51525...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3c(oc4cc(O)cc(O)c4c3=O)-c3ccc(O)cc3)c2o1 |(4.11,-9.88,;3.39,-11.25,;4.21,-12.55,;3.49,-13.91,;1.94,-13.97,;1.13,-12.67,;1.84,-11.31,;1.22,-15.32,;2.03,-16.61,;1.32,-17.95,;2.13,-19.25,;-.19,-18,;-.92,-19.36,;-.11,-20.67,;-2.45,-19.41,;-3.25,-18.11,;-4.31,-19.19,;-2.52,-16.76,;-3.32,-15.46,;-3.32,-13.91,;-4.67,-13.14,;-6,-13.93,;-7.34,-13.16,;-8.67,-13.94,;-10.01,-13.17,;-8.67,-15.48,;-7.34,-16.25,;-7.34,-17.8,;-6,-15.48,;-4.66,-16.24,;-4.56,-17.65,;-2.16,-12.91,;-.7,-13.43,;.47,-12.42,;.18,-10.91,;1.35,-9.9,;-1.28,-10.4,;-2.45,-11.41,;-1,-16.72,;-.3,-15.38,)| Show InChI InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)22-12-21(37)24-19(35)11-20(36)26(30(24)39-22)27-28(38)25-18(34)9-17(33)10-23(25)40-29(27)14-3-7-16(32)8-4-14/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50323212
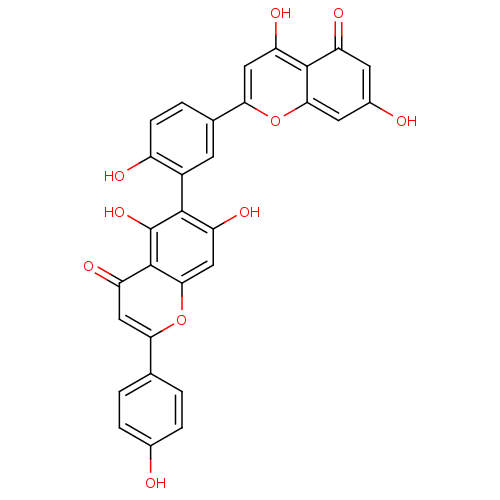
(6-[5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydro...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)c(c(O)cc2o1)-c1cc(ccc1O)-c1cc(O)c2c(cc(O)cc2=O)o1 |(14.56,-27.41,;13.23,-26.63,;11.89,-27.39,;10.56,-26.61,;10.57,-25.07,;11.9,-24.31,;13.23,-25.08,;9.25,-24.3,;7.91,-25.06,;6.59,-24.28,;5.25,-25.04,;6.6,-22.75,;5.27,-21.99,;3.93,-22.76,;5.28,-20.46,;6.6,-19.69,;6.6,-18.15,;7.93,-20.45,;7.93,-21.99,;9.26,-22.76,;3.95,-19.69,;2.61,-20.46,;1.28,-19.68,;1.28,-18.14,;2.61,-17.37,;3.95,-18.15,;5.29,-17.38,;-.06,-20.44,;-.06,-22,;-1.41,-22.77,;-1.41,-24.31,;-2.75,-21.99,;-2.75,-20.44,;-4.08,-19.67,;-5.41,-20.45,;-6.75,-19.67,;-5.42,-21.99,;-4.08,-22.76,;-4.08,-24.3,;-1.41,-19.66,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)23-11-22(37)29-26(39-23)12-20(35)27(30(29)38)17-7-14(3-6-18(17)33)24-10-21(36)28-19(34)8-16(32)9-25(28)40-24/h1-12,31-33,35-36,38H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060921

(CHEMBL3394770)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)\c1c(ccc(-[#6]-[#8])c1C#Cc1ccc(-[#8])cc1)-c1ccc(-[#8])cc1 |c:11,15| Show InChI InChI=1S/C35H26O5/c1-40-31-18-9-26(10-19-31)34(25-7-16-30(39)17-8-25)35-32(24-5-14-29(38)15-6-24)21-11-27(22-36)33(35)20-4-23-2-12-28(37)13-3-23/h2-3,5-19,21,36-38H,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060920

(CHEMBL3394771)Show SMILES OCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C34H26O7/c35-20-22-4-17-31(30-18-15-28(39)19-32(30)40)33(29(22)16-3-21-1-9-25(36)10-2-21)34(41,23-5-11-26(37)12-6-23)24-7-13-27(38)14-8-24/h1-2,4-15,17-19,35-41H,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093524
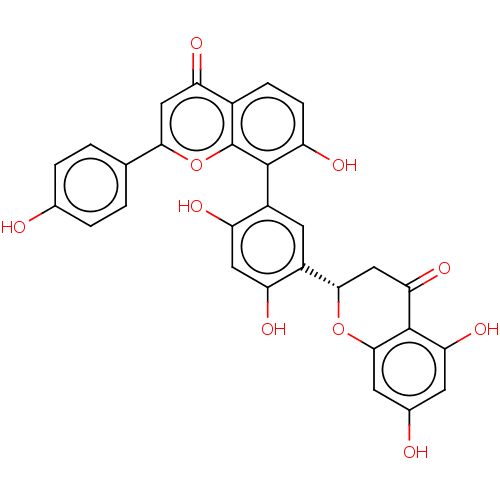
(CHEMBL3585680)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(O)c(-c3cc([C@@H]4CC(=O)c5c(O)cc(O)cc5O4)c(O)cc3O)c2o1 |r,wD:20.20,(1.56,-5.97,;2.63,-5.36,;3.96,-6.14,;5.3,-5.38,;5.31,-3.84,;3.98,-3.06,;2.64,-3.82,;6.65,-3.07,;7.97,-3.85,;9.31,-3.09,;10.37,-3.71,;9.32,-1.55,;10.65,-.79,;10.67,.76,;9.34,1.54,;9.34,2.77,;8,.76,;6.66,1.53,;5.33,.77,;4,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-3.75,1.39,;-1.33,1.54,;,.77,;1.33,1.54,;4,3.08,;2.93,3.7,;5.33,3.85,;6.67,3.07,;7.73,3.69,;7.99,-.78,;6.65,-1.54,)| Show InChI InChI=1S/C30H20O10/c31-14-3-1-13(2-4-14)25-11-22(36)16-5-6-19(33)28(30(16)40-25)18-9-17(20(34)10-21(18)35)26-12-24(38)29-23(37)7-15(32)8-27(29)39-26/h1-11,26,31-35,37H,12H2/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50323213
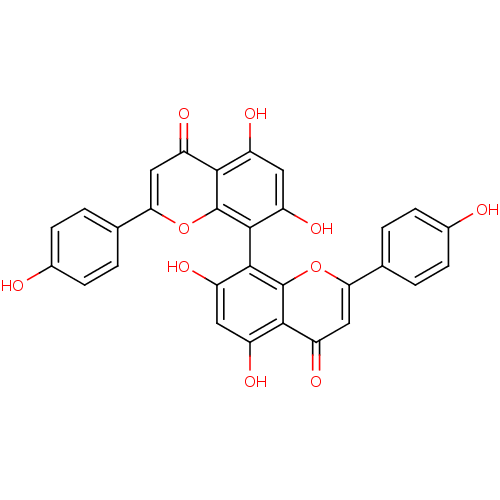
(CHEMBL1208973 | cupressuflavone)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3c(O)cc(O)c4c3oc(cc4=O)-c3ccc(O)cc3)c2o1 |(5.18,-39.53,;3.84,-40.3,;3.85,-41.86,;2.51,-42.63,;1.18,-41.86,;1.18,-40.31,;2.51,-39.54,;-.15,-42.62,;-.15,-44.15,;-1.48,-44.92,;-1.48,-46.46,;-2.8,-44.15,;-4.14,-44.93,;-4.14,-46.48,;-5.47,-44.16,;-5.47,-42.62,;-6.8,-41.84,;-4.13,-41.86,;-4.13,-35.97,;-5.46,-35.2,;-6.8,-35.97,;-5.46,-33.67,;-4.13,-32.9,;-4.13,-31.36,;-2.8,-33.65,;-2.79,-35.2,;-1.45,-35.97,;-.11,-35.19,;-.12,-33.64,;-1.46,-32.87,;-1.46,-31.33,;1.21,-35.96,;1.22,-37.5,;2.55,-38.27,;3.88,-37.5,;5.22,-38.27,;3.88,-35.96,;2.55,-35.19,;-2.8,-42.62,;-1.48,-41.85,)| Show InChI InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)23-11-21(37)25-17(33)9-19(35)27(29(25)39-23)28-20(36)10-18(34)26-22(38)12-24(40-30(26)28)14-3-7-16(32)8-4-14/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060918
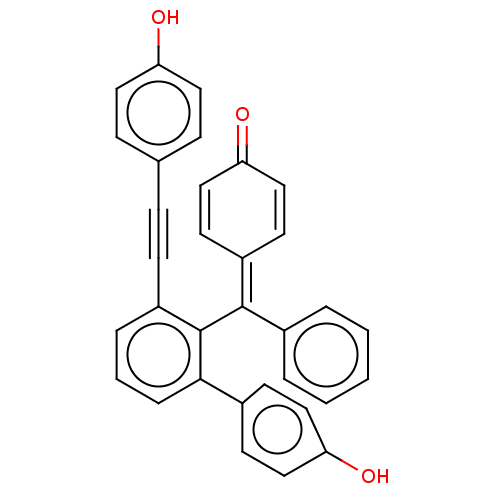
(Selaginellin)Show SMILES [#8]-c1ccc(cc1)C#Cc1cccc(-c2ccc(-[#8])cc2)c1\[#6](=[#6]-1/[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1 |c:27,31| Show InChI InChI=1S/C33H22O3/c34-28-17-10-23(11-18-28)9-12-26-7-4-8-31(24-13-19-29(35)20-14-24)33(26)32(25-5-2-1-3-6-25)27-15-21-30(36)22-16-27/h1-8,10-11,13-22,34-35H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093523
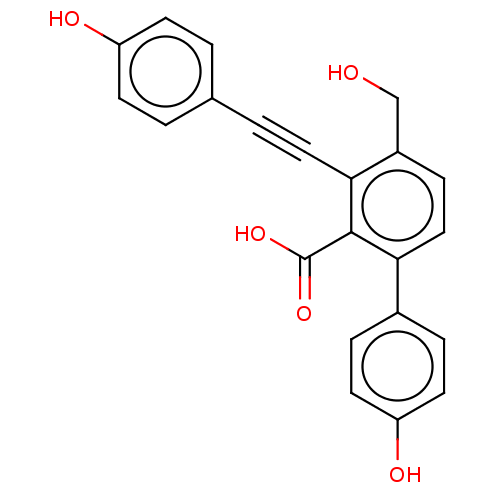
(CHEMBL3585679 | PTP1B spring 6 (6))Show SMILES OCc1ccc(-c2ccc(O)cc2)c(C(O)=O)c1C#Cc1ccc(O)cc1 Show InChI InChI=1S/C22H16O5/c23-13-16-6-12-19(15-4-9-18(25)10-5-15)21(22(26)27)20(16)11-3-14-1-7-17(24)8-2-14/h1-2,4-10,12,23-25H,13H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060919

(CHEMBL3394772)Show SMILES COCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C35H28O7/c1-42-21-23-5-18-32(31-19-16-29(39)20-33(31)40)34(30(23)17-4-22-2-10-26(36)11-3-22)35(41,24-6-12-27(37)13-7-24)25-8-14-28(38)15-9-25/h2-3,5-16,18-20,36-41H,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433414
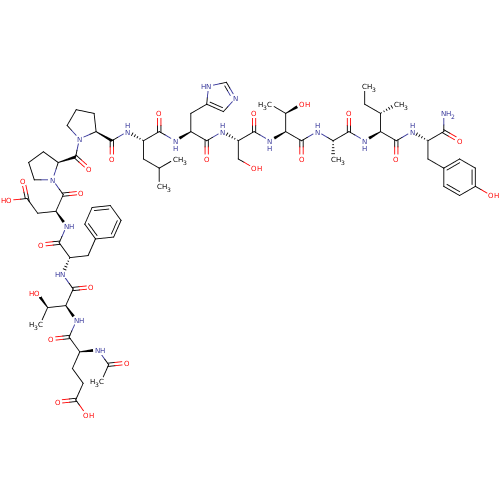
(CHEMBL2380650)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(C)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C71H102N16O22/c1-9-36(4)56(67(105)77-46(59(72)97)28-42-19-21-44(92)22-20-42)83-60(98)37(5)75-68(106)57(38(6)89)85-65(103)51(33-88)82-64(102)49(30-43-32-73-34-74-43)78-62(100)47(27-35(2)3)79-66(104)52-17-13-25-86(52)71(109)53-18-14-26-87(53)70(108)50(31-55(95)96)81-63(101)48(29-41-15-11-10-12-16-41)80-69(107)58(39(7)90)84-61(99)45(76-40(8)91)23-24-54(93)94/h10-12,15-16,19-22,32,34-39,45-53,56-58,88-90,92H,9,13-14,17-18,23-31,33H2,1-8H3,(H2,72,97)(H,73,74)(H,75,106)(H,76,91)(H,77,105)(H,78,100)(H,79,104)(H,80,107)(H,81,101)(H,82,102)(H,83,98)(H,84,99)(H,85,103)(H,93,94)(H,95,96)/t36-,37-,38+,39+,45-,46-,47-,48-,49-,50-,51-,52-,53-,56-,57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060923
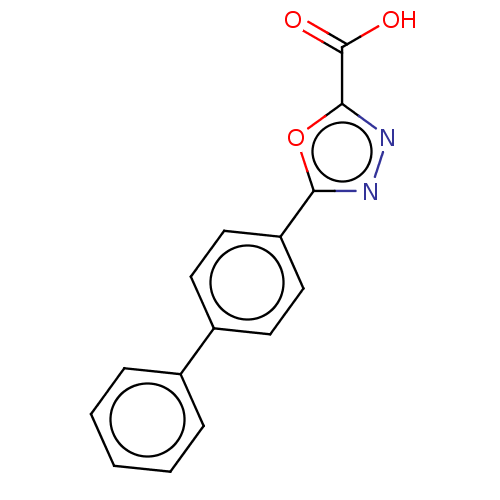
(CHEMBL3394773)Show InChI InChI=1S/C15H10N2O3/c18-15(19)14-17-16-13(20-14)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433415
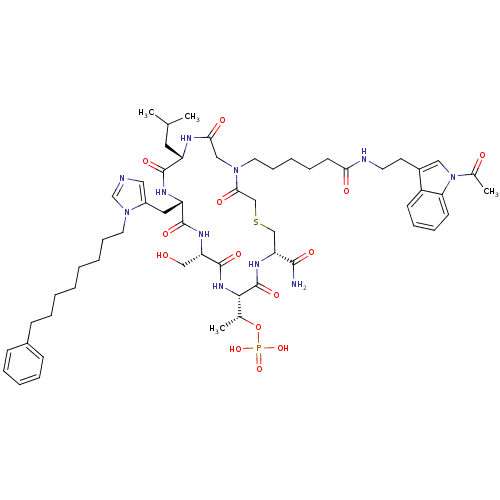
(CHEMBL2380764)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCCCCC(=O)NCCc2cn(C(C)=O)c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cncn2CCCCCCCCc2ccccc2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C58H84N11O14PS/c1-38(2)29-45-55(76)63-46(30-43-31-60-37-68(43)28-17-8-6-5-7-11-19-41-20-12-9-13-21-41)56(77)64-47(34-70)57(78)66-53(39(3)83-84(80,81)82)58(79)65-48(54(59)75)35-85-36-52(74)67(33-51(73)62-45)27-18-10-14-24-50(72)61-26-25-42-32-69(40(4)71)49-23-16-15-22-44(42)49/h9,12-13,15-16,20-23,31-32,37-39,45-48,53,70H,5-8,10-11,14,17-19,24-30,33-36H2,1-4H3,(H2,59,75)(H,61,72)(H,62,73)(H,63,76)(H,64,77)(H,65,79)(H,66,78)(H2,80,81,82)/t39-,45+,46+,47+,48-,53+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433416
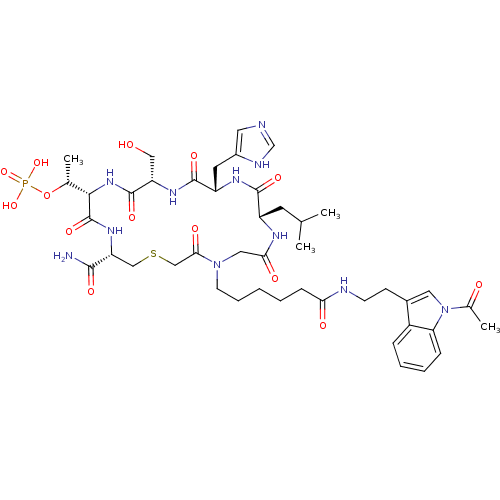
(CHEMBL2380660)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCCCCC(=O)NCCc2cn(C(C)=O)c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C44H64N11O14PS/c1-25(2)16-31-41(62)50-32(17-29-18-46-24-48-29)42(63)51-33(21-56)43(64)53-39(26(3)69-70(66,67)68)44(65)52-34(40(45)61)22-71-23-38(60)54(20-37(59)49-31)15-9-5-6-12-36(58)47-14-13-28-19-55(27(4)57)35-11-8-7-10-30(28)35/h7-8,10-11,18-19,24-26,31-34,39,56H,5-6,9,12-17,20-23H2,1-4H3,(H2,45,61)(H,46,48)(H,47,58)(H,49,59)(H,50,62)(H,51,63)(H,52,65)(H,53,64)(H2,66,67,68)/t26-,31+,32+,33+,34-,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093526
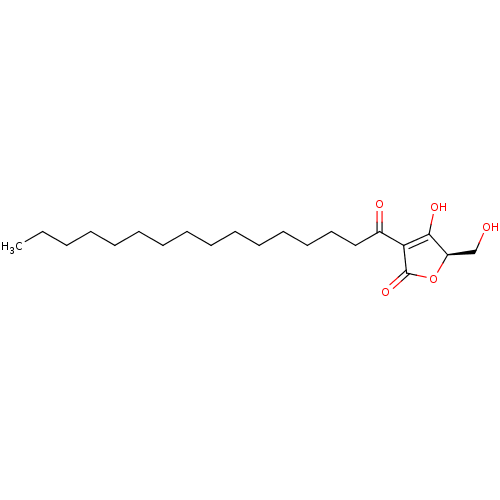
(CHEMBL426373 | RK-682)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(O)[C@@H](CO)OC1=O |r,c:17| Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h18,22,24H,2-16H2,1H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50259862

(13,II8-biapigenin | 3,8''-biapigenin | CHEMBL51525...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3c(oc4cc(O)cc(O)c4c3=O)-c3ccc(O)cc3)c2o1 |(4.11,-9.88,;3.39,-11.25,;4.21,-12.55,;3.49,-13.91,;1.94,-13.97,;1.13,-12.67,;1.84,-11.31,;1.22,-15.32,;2.03,-16.61,;1.32,-17.95,;2.13,-19.25,;-.19,-18,;-.92,-19.36,;-.11,-20.67,;-2.45,-19.41,;-3.25,-18.11,;-4.31,-19.19,;-2.52,-16.76,;-3.32,-15.46,;-3.32,-13.91,;-4.67,-13.14,;-6,-13.93,;-7.34,-13.16,;-8.67,-13.94,;-10.01,-13.17,;-8.67,-15.48,;-7.34,-16.25,;-7.34,-17.8,;-6,-15.48,;-4.66,-16.24,;-4.56,-17.65,;-2.16,-12.91,;-.7,-13.43,;.47,-12.42,;.18,-10.91,;1.35,-9.9,;-1.28,-10.4,;-2.45,-11.41,;-1,-16.72,;-.3,-15.38,)| Show InChI InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)22-12-21(37)24-19(35)11-20(36)26(30(24)39-22)27-28(38)25-18(34)9-17(33)10-23(25)40-29(27)14-3-7-16(32)8-4-14/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060922

(CHEMBL3394769)Show SMILES [#8]-[#6]-c1ccc(-c2ccc(-[#8])cc2)c(\[#6](=[#6]-2/[#6]=[#6]-[#6](=O)-[#6]=[#6]-2)-c2ccc(-[#8])cc2)c1C#Cc1ccc(-[#8])cc1 |c:17,21| Show InChI InChI=1S/C34H24O5/c35-21-26-10-20-31(23-4-13-28(37)14-5-23)34(32(26)19-3-22-1-11-27(36)12-2-22)33(24-6-15-29(38)16-7-24)25-8-17-30(39)18-9-25/h1-2,4-18,20,35-38H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433413
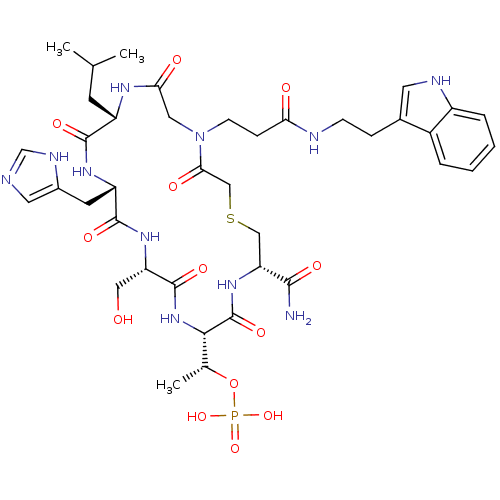
(CHEMBL2380651)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCC(=O)NCCc2c[nH]c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C39H56N11O13PS/c1-21(2)12-27-36(56)46-28(13-24-15-41-20-44-24)37(57)47-29(17-51)38(58)49-34(22(3)63-64(60,61)62)39(59)48-30(35(40)55)18-65-19-33(54)50(16-32(53)45-27)11-9-31(52)42-10-8-23-14-43-26-7-5-4-6-25(23)26/h4-7,14-15,20-22,27-30,34,43,51H,8-13,16-19H2,1-3H3,(H2,40,55)(H,41,44)(H,42,52)(H,45,53)(H,46,56)(H,47,57)(H,48,59)(H,49,58)(H2,60,61,62)/t22-,27+,28+,29+,30-,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093525

(taiwaniaflavone)Show SMILES Oc1ccc(cc1)-c1oc2cc(O)cc(O)c2c(=O)c1-c1cc(ccc1O)-c1cc(=O)c2c(O)cc(O)cc2o1 |(2.2,-12.35,;.87,-13.13,;.88,-14.67,;-.45,-15.44,;-1.79,-14.67,;-1.8,-13.14,;-.47,-12.36,;-3.12,-15.45,;-4.47,-14.67,;-5.81,-15.46,;-7.15,-14.7,;-8.47,-15.47,;-9.81,-14.7,;-8.48,-17.01,;-7.14,-17.78,;-7.15,-19.32,;-5.8,-17.02,;-4.46,-17.78,;-4.45,-19.32,;-3.11,-17,;-1.78,-17.77,;-.46,-17,;.88,-17.76,;.89,-19.3,;-.45,-20.08,;-1.78,-19.31,;-3.11,-20.08,;2.21,-16.98,;2.19,-15.44,;3.52,-14.67,;3.52,-13.13,;4.86,-15.43,;6.18,-14.67,;6.17,-13.13,;7.51,-15.42,;7.52,-16.96,;8.86,-17.73,;6.19,-17.73,;4.86,-16.97,;3.54,-17.75,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)30-26(29(38)28-21(36)9-17(33)11-25(28)40-30)18-7-14(3-6-19(18)34)23-12-22(37)27-20(35)8-16(32)10-24(27)39-23/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50323212

(6-[5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydro...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)c(c(O)cc2o1)-c1cc(ccc1O)-c1cc(O)c2c(cc(O)cc2=O)o1 |(14.56,-27.41,;13.23,-26.63,;11.89,-27.39,;10.56,-26.61,;10.57,-25.07,;11.9,-24.31,;13.23,-25.08,;9.25,-24.3,;7.91,-25.06,;6.59,-24.28,;5.25,-25.04,;6.6,-22.75,;5.27,-21.99,;3.93,-22.76,;5.28,-20.46,;6.6,-19.69,;6.6,-18.15,;7.93,-20.45,;7.93,-21.99,;9.26,-22.76,;3.95,-19.69,;2.61,-20.46,;1.28,-19.68,;1.28,-18.14,;2.61,-17.37,;3.95,-18.15,;5.29,-17.38,;-.06,-20.44,;-.06,-22,;-1.41,-22.77,;-1.41,-24.31,;-2.75,-21.99,;-2.75,-20.44,;-4.08,-19.67,;-5.41,-20.45,;-6.75,-19.67,;-5.42,-21.99,;-4.08,-22.76,;-4.08,-24.3,;-1.41,-19.66,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)23-11-22(37)29-26(39-23)12-20(35)27(30(29)38)17-7-14(3-6-18(17)33)24-10-21(36)28-19(34)8-16(32)9-25(28)40-24/h1-12,31-33,35-36,38H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433417
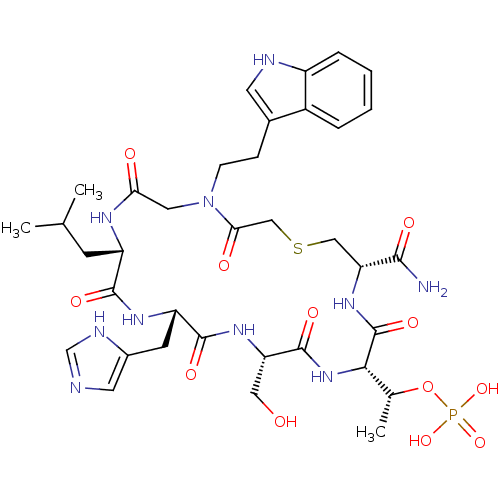
(CHEMBL2380653)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCc2c[nH]c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C36H51N10O12PS/c1-19(2)10-25-33(51)42-26(11-22-13-38-18-40-22)34(52)43-27(15-47)35(53)45-31(20(3)58-59(55,56)57)36(54)44-28(32(37)50)16-60-17-30(49)46(14-29(48)41-25)9-8-21-12-39-24-7-5-4-6-23(21)24/h4-7,12-13,18-20,25-28,31,39,47H,8-11,14-17H2,1-3H3,(H2,37,50)(H,38,40)(H,41,48)(H,42,51)(H,43,52)(H,44,54)(H,45,53)(H2,55,56,57)/t20-,25+,26+,27+,28-,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50323213

(CHEMBL1208973 | cupressuflavone)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3c(O)cc(O)c4c3oc(cc4=O)-c3ccc(O)cc3)c2o1 |(5.18,-39.53,;3.84,-40.3,;3.85,-41.86,;2.51,-42.63,;1.18,-41.86,;1.18,-40.31,;2.51,-39.54,;-.15,-42.62,;-.15,-44.15,;-1.48,-44.92,;-1.48,-46.46,;-2.8,-44.15,;-4.14,-44.93,;-4.14,-46.48,;-5.47,-44.16,;-5.47,-42.62,;-6.8,-41.84,;-4.13,-41.86,;-4.13,-35.97,;-5.46,-35.2,;-6.8,-35.97,;-5.46,-33.67,;-4.13,-32.9,;-4.13,-31.36,;-2.8,-33.65,;-2.79,-35.2,;-1.45,-35.97,;-.11,-35.19,;-.12,-33.64,;-1.46,-32.87,;-1.46,-31.33,;1.21,-35.96,;1.22,-37.5,;2.55,-38.27,;3.88,-37.5,;5.22,-38.27,;3.88,-35.96,;2.55,-35.19,;-2.8,-42.62,;-1.48,-41.85,)| Show InChI InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)23-11-21(37)25-17(33)9-19(35)27(29(25)39-23)28-20(36)10-18(34)26-22(38)12-24(40-30(26)28)14-3-7-16(32)8-4-14/h1-12,31-36H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 ... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093524

(CHEMBL3585680)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(O)c(-c3cc([C@@H]4CC(=O)c5c(O)cc(O)cc5O4)c(O)cc3O)c2o1 |r,wD:20.20,(1.56,-5.97,;2.63,-5.36,;3.96,-6.14,;5.3,-5.38,;5.31,-3.84,;3.98,-3.06,;2.64,-3.82,;6.65,-3.07,;7.97,-3.85,;9.31,-3.09,;10.37,-3.71,;9.32,-1.55,;10.65,-.79,;10.67,.76,;9.34,1.54,;9.34,2.77,;8,.76,;6.66,1.53,;5.33,.77,;4,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-3.75,1.39,;-1.33,1.54,;,.77,;1.33,1.54,;4,3.08,;2.93,3.7,;5.33,3.85,;6.67,3.07,;7.73,3.69,;7.99,-.78,;6.65,-1.54,)| Show InChI InChI=1S/C30H20O10/c31-14-3-1-13(2-4-14)25-11-22(36)16-5-6-19(33)28(30(16)40-25)18-9-17(20(34)10-21(18)35)26-12-24(38)29-23(37)7-15(32)8-27(29)39-26/h1-11,26,31-35,37H,12H2/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 2... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433418
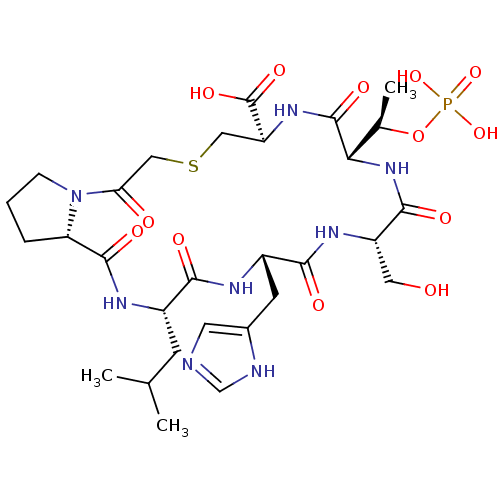
(CHEMBL2380765)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H45N8O13PS/c1-14(2)7-17-24(40)32-18(8-16-9-30-13-31-16)25(41)34-19(10-38)26(42)36-23(15(3)50-51(47,48)49)28(44)35-20(29(45)46)11-52-12-22(39)37-6-4-5-21(37)27(43)33-17/h9,13-15,17-21,23,38H,4-8,10-12H2,1-3H3,(H,30,31)(H,32,40)(H,33,43)(H,34,41)(H,35,44)(H,36,42)(H,45,46)(H2,47,48,49)/t15-,17+,18+,19+,20+,21+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060921

(CHEMBL3394770)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)\c1c(ccc(-[#6]-[#8])c1C#Cc1ccc(-[#8])cc1)-c1ccc(-[#8])cc1 |c:11,15| Show InChI InChI=1S/C35H26O5/c1-40-31-18-9-26(10-19-31)34(25-7-16-30(39)17-8-25)35-32(24-5-14-29(38)15-6-24)21-11-27(22-36)33(35)20-4-23-2-12-28(37)13-3-23/h2-3,5-19,21,36-38H,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093523

(CHEMBL3585679 | PTP1B spring 6 (6))Show SMILES OCc1ccc(-c2ccc(O)cc2)c(C(O)=O)c1C#Cc1ccc(O)cc1 Show InChI InChI=1S/C22H16O5/c23-13-16-6-12-19(15-4-9-18(25)10-5-15)21(22(26)27)20(16)11-3-14-1-7-17(24)8-2-14/h1-2,4-10,12,23-25H,13H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... |
Bioorg Med Chem 23: 3730-7 (2015)
Article DOI: 10.1016/j.bmc.2015.04.007
BindingDB Entry DOI: 10.7270/Q25T3N81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060918

(Selaginellin)Show SMILES [#8]-c1ccc(cc1)C#Cc1cccc(-c2ccc(-[#8])cc2)c1\[#6](=[#6]-1/[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1 |c:27,31| Show InChI InChI=1S/C33H22O3/c34-28-17-10-23(11-18-28)9-12-26-7-4-8-31(24-13-19-29(35)20-14-24)33(26)32(25-5-2-1-3-6-25)27-15-21-30(36)22-16-27/h1-8,10-11,13-22,34-35H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060919

(CHEMBL3394772)Show SMILES COCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C35H28O7/c1-42-21-23-5-18-32(31-19-16-29(39)20-33(31)40)34(30(23)17-4-22-2-10-26(36)11-3-22)35(41,24-6-12-27(37)13-7-24)25-8-14-28(38)15-9-25/h2-3,5-16,18-20,36-41H,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060920

(CHEMBL3394771)Show SMILES OCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C34H26O7/c35-20-22-4-17-31(30-18-15-28(39)19-32(30)40)33(29(22)16-3-21-1-9-25(36)10-2-21)34(41,23-5-11-26(37)12-6-23)24-7-13-27(38)14-8-24/h1-2,4-15,17-19,35-41H,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50426931
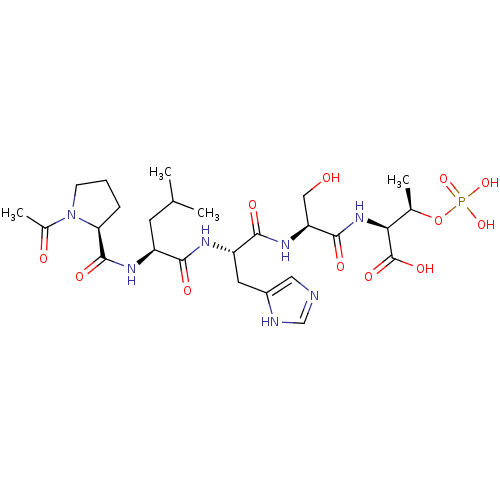
(CHEMBL2323729 | US20230365568, Compound 6a)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)OP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H42N7O12P/c1-13(2)8-17(30-25(39)20-6-5-7-33(20)15(4)35)22(36)29-18(9-16-10-27-12-28-16)23(37)31-19(11-34)24(38)32-21(26(40)41)14(3)45-46(42,43)44/h10,12-14,17-21,34H,5-9,11H2,1-4H3,(H,27,28)(H,29,36)(H,30,39)(H,31,37)(H,32,38)(H,40,41)(H2,42,43,44)/t14-,17+,18+,19+,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data